| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 12, December 2023, pages 400-404
Synchronous Occurrence of Triple-Negative Breast Cancer and Malignant Melanoma
Margarita Stoyanova Taushanovaa, Yoana Ivanova Milushevab, Dimo Angelov Manova, Ralitza Rosen Hadjievaa, Angel Danchev Yordanovc, d
aDepartment of Medical Oncology, MBAL “Nadezhda Hospital”, 1000 Sofia, Bulgaria
bDepartment of Medical Oncology, University Hospital “Tsaritsa Yoanna-ISUL”, 1000 Sofia, Bulgaria
cDepartment of Gynaecological Oncology, Medical University Pleven, 5800 Pleven, Bulgaria
dCorresponding Author: Angel Yordanov, Department of Gynaecological Oncology, Medical University Pleven, 5800 Pleven, Bulgaria
Manuscript submitted November 2, 2023, accepted November 30, 2023, published online December 29, 2023
Short title: Synchronous Breast Cancer and Melanoma
doi: https://doi.org/10.14740/jmc4167
| Abstract | ▴Top |
In people with cancer, multiple primary malignant neoplasms (MPMNs) are not unusual, and they may be caused by risk factors such as genetics, viral infection, smoking, environmental factors, or treatment-related variables. The frequency of MPMNs occurring in the same or separate organ systems is between 2% and 17%. The 5-year breast cancer survivors have been found to have around 3.6% chance of acquiring another neoplasm. In this case report, we present a very rare simultaneous occurrence of two highly malignant tumors - triple-negative breast cancer and cutaneous melanoma. We performed genetic tests for determining the link between both neoplasms. The patient was treated in an adjuvant setting with chemotherapy and immunotherapy with pembrolizumab. According to epidemiological studies, for primary cutaneous melanoma following breast cancer, the standardized incidence ratio (SIR) varied from 1.03 to 4.10, while for primary breast carcinoma following cutaneous melanoma, it varied from 1.16 to 5.13. A number of risk factors have been proven to increase the risk of a second primary malignancy. This case highlights the importance of risk factor assessment and thorough primary workup of each patient. It emphasizes the need for a personalized approach when treating synchronous neoplasms.
Keywords: Synchronous tumors; Metachronous malignancies; Triple-negative breast cancer; Malignant melanoma
| Introduction | ▴Top |
Due to the massive advancement of diagnostic techniques and treatment options for different malignancies, the overall survival rate of patients diagnosed with cancer has increased significantly and this increases the risk of acquiring another neoplasm. Cancer patients have a 20% higher risk of developing another primary cancer than the general population. Also, the development of a second tumor is becoming the leading cause of death among long-term cancer survivors.
Multiple primary malignant neoplasms (MPMNs) are defined as two or more malignancies that arise independently of each other in the same or different organ [1, 2]. Synchronous tumors (SMPMNs) develop almost simultaneously. The incidence of MPMNs ranges from 3% to 5%.
Epidemiological studies suggest that following a breast cancer (BC) diagnosis among survivors at 5, 10, and 15 years, there is a 3.6%, 8.2% and 13.9% chance of acquiring another neoplasm, respectively [3].
Triple-negative breast cancer (TNBC) refers to BC patients with negative estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2). TNBCs tend to grow and spread more quickly than other types of BC. Because of this, TNBC is considered to be more aggressive than other forms of BC.
About 70% of BCs diagnosed in women with an inherited BRCA1 mutation are TNBC. Chemotherapy and immunotherapy are the primary treatment options for TNBC [2, 4].
The development of malignant melanoma and its treatment is a challenge for both adjuvant treatment and metastatic disease. Melanoma is a rare but exceptionally malignant disease. Its early diagnosis is the way to its successful treatment.
Neoadjuvant chemotherapy and immunotherapy have been proven effective for BC in KEYNOTE-522 trial. The treatment with pembrolizumab in stage II B after radical treatment achieves a significant effect, confirmed by the clinical trial KEYNOTE-716 [5-7].
When diagnosing synchronous tumors, the correct assessment of their treatment is related to their good results. The right choice of treatment relies on the accurate staging of each tumor and the individual approach toward each patient.
In this case report, we will discuss our experience treating a patient with synchronous tumors - TNBC and melanoma. Their simultaneous diagnosis emphasizes that early detection and a multidisciplinary approach are crucial for the successful treatment of MPMNs. Conducting highly specific genetic tests could help us find the most appropriate treatment, especially in the presence of synchronous tumors. Conducting adjuvant therapy according to the stages of both tumors would lead to better treatment results.
| Case Report | ▴Top |
Investigations
A 59-year-old postmenopausal woman was diagnosed with BC. The clinical staging indicated a localized tumor (cT1) with no lymph node involvement (cN0) and no distant metastases on CT scan (cM0) with tumor marker (CA15-3) within the normal range. Breast-conserving surgery was performed in July 2022, and the histological analysis revealed invasive ductal grade 3 BC, pT1pN1M0, ER-, PR-, HER2-, Ki-67 of 50% (Fig. 1).
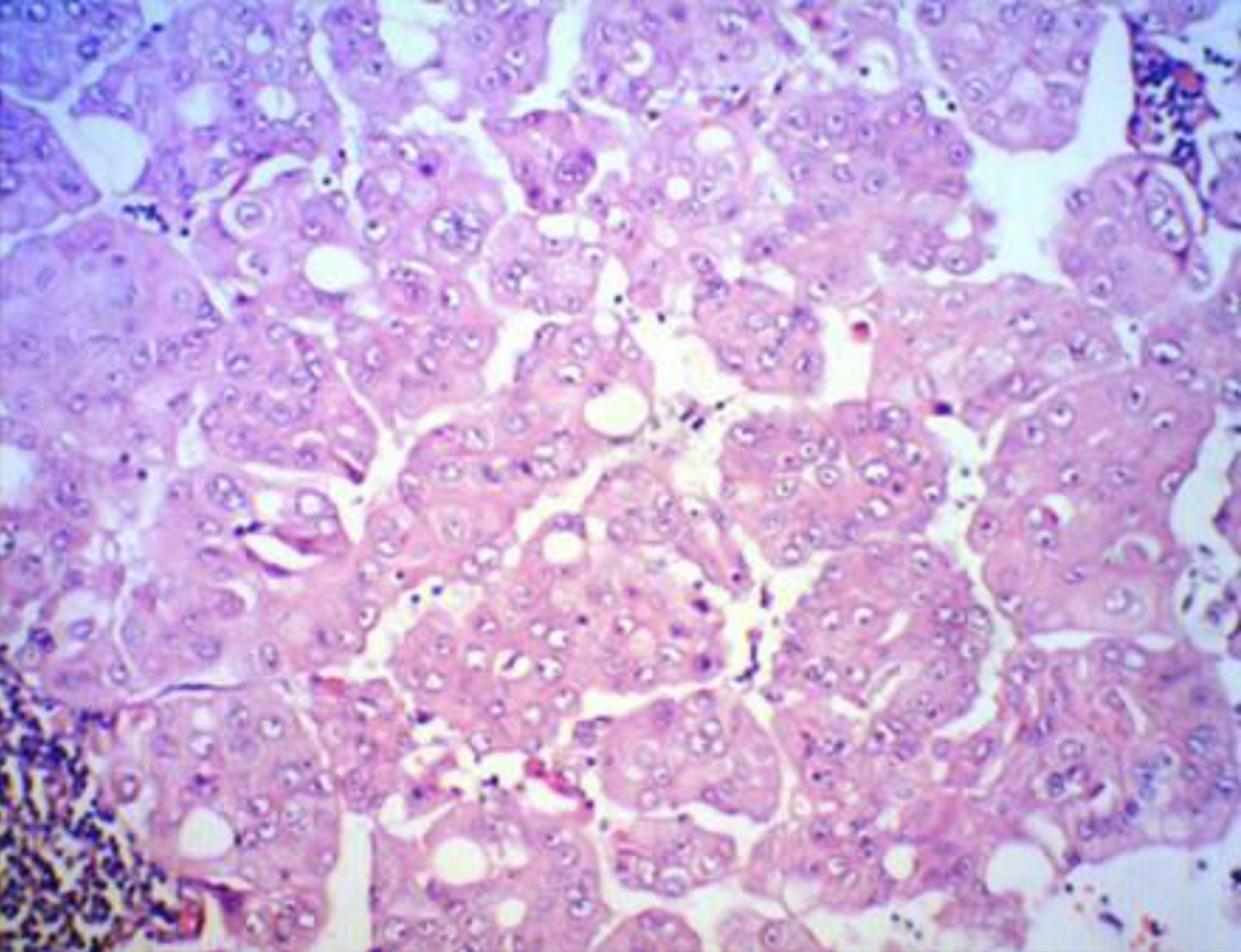 Click for large image | Figure 1. TNBC histological fundings XE, × 100. |
Diagnosis
During her hospitalization, a pigmented lesion was found on the front surface of the right thigh. In August 2022, a single-photon emission computed tomography (SPECT)/computed tomography (CT) was performed which revealed three sentinel lymph nodes around the inguinal ligament, cranially directed to the primary lesion drainage path, marked on the patient’s skin with blue dye. Three nonenlarged sentinel lymph nodes in a caudal direction in the right fossa poplitea were also described. The same month, resection of the primary skin lesion followed, as well as sentinel dissection of the described lymph nodes. Histopathological examination showed a superficial and nodular malignant melanoma Clark 3, Breslow 3 mm, with ulceration, high mitotic activity, and profuse lymphocytic stromal reaction (Fig. 2). It shows clear surgical margins without lymph node involvement, final staging - pT3bpN0M0 R0, melan-A+, S-100+, CKAE1/AE3+, SOX10+, Ki-67 of 50%, lactate dehydrogenase (LDH) 185 U/L, S-100 0.07 µg/L after surgical treatment.
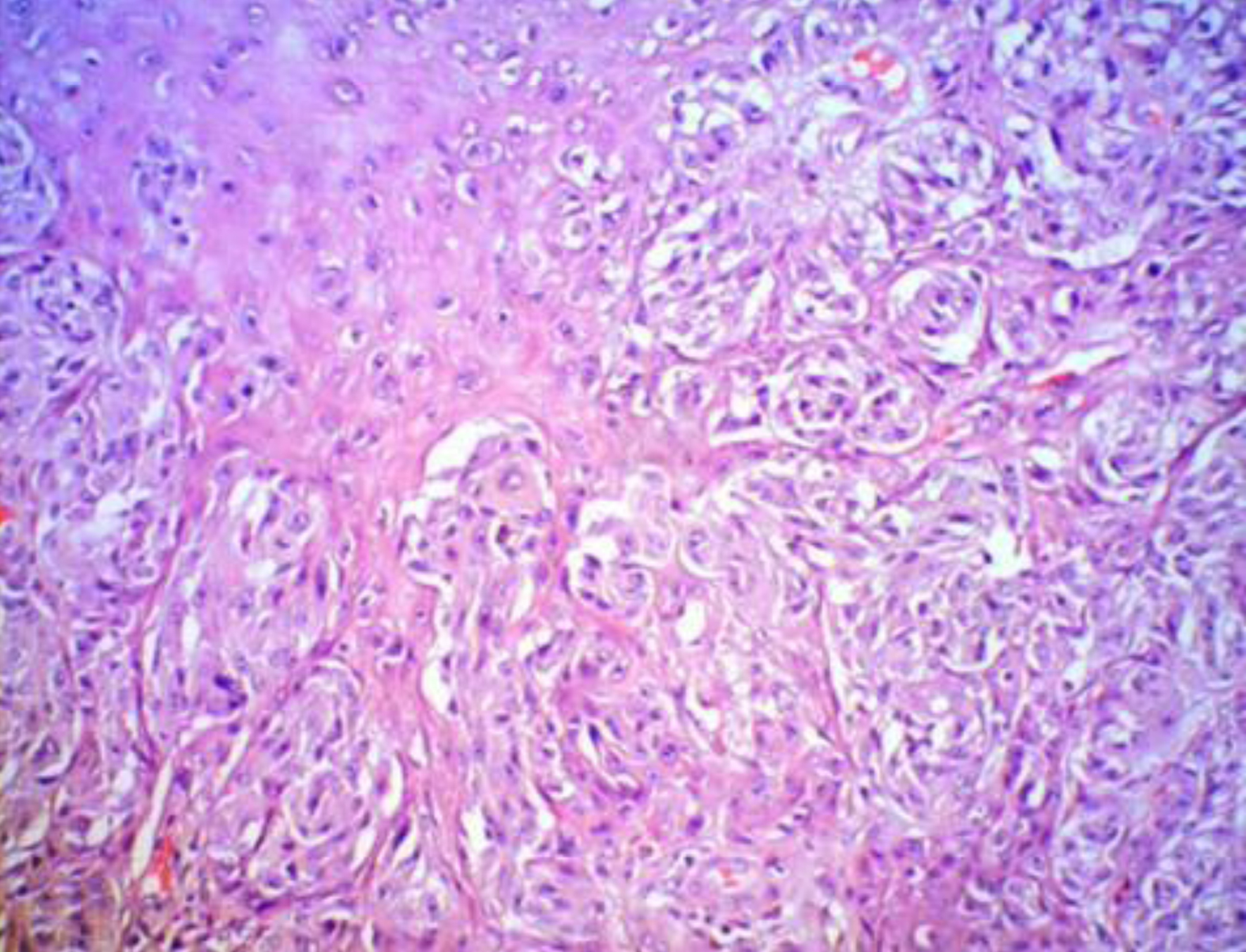 Click for large image | Figure 2. Melanoma histological fundings XE, × 100. |
Hematological investigations were within normal limits. ECOG was 0 and there was no deviation on physical examination. The BRCA 1/2 test was negative. FoundationOne®Liquid CDx was performed (Table 1). She had a family history of malignancies: her father died of pancreatic cancer and her mother died of colon cancer.
 Click to view | Table 1. FoundationOne/Liquid CDx Results |
Treatment
The patient was considered for systemic therapy. On October 10, 2022, the patient was started on dose-dense chemotherapy: four cycles of epirubicin + cyclophosphamide, followed by 12 weekly infusions of paclitaxel 80 mg/m2 + carboplatin AUC 1.5 + pembrolizumab 200 mg. Following the adjuvant therapy, radiotherapy was conducted with a whole breast dose of 50 Gy.
Follow-up and outcomes
The patient continues immunotherapy with pembrolizumab and no adverse events were registered. CT scan was conducted in April 2023 with no evidence of recurrence or distant metastases.
| Discussion | ▴Top |
Malignancies that are identified and histologically verified in the same host simultaneously or within a 6-month period after one another are referred to as synchronous malignancies. Common etiological variables including smoking (lung, head, and neck, bladder, etc.), genetics (Cowden syndrome, von-Hippel-Lindau disease), and hormones (multiple endocrine neoplasia, BC, endometrial cancer) are thought to be responsible for their simultaneous occurrence. When a person has MPMNs, there are usually similar or related etiological variables to blame. There have been reports of correlations between BC and other cancers in women, including ovarian cancer and melanoma.
The most frequent cancer in women is BC, which also ranks second globally in terms of cancer-related death [8]. Roughly, 15% to 20% of all cases of BC are TNBC [9]. TNBC has a worse prognosis than BCs with positive hormone receptor status. In the first 3 - 5 years following diagnosis, over 50% of patients relapse [10, 11], and the median overall survival (OS) at present therapy is 10.2 months [12]. The subtype of TNBC best responds to conventional chemotherapy regimens: taxanes or anthracyclines. Less than 30% of TNBC patients, however, experience a complete response, and even then their mortality and recurrence rates are still greater than those of non-TNBC subtypes. Furthermore, it has been shown that immunotherapy enhances response and overall survival in TNBC patients.
The current “golden” standard for high-risk patients with early TNBC is neoadjuvant pembrolizumab plus chemotherapy and adjuvant pembrolizumab monotherapy. Patients with tumors larger than 2 cm or with positive lymph nodes at presentation are eligible for this treatment according to the KEYNOTE-522 study [8]. Patients included in the trial received neoadjuvant therapy with pembrolizumab (at a dose of 200 mg) plus paclitaxel and carboplatin and continued with an anthracycline regimen for four cycles. The patients had adjuvant pembrolizumab or placebo every 3 weeks for a maximum of nine cycles, following final surgery [7]. Both the pathologic complete response (pCR) and the event-free survival (EFS) primary objectives were achieved by the study. The pembrolizumab-treated patients had a pCR rate of 64.8%, while the placebo-treated patients’ pCR rate was 51.2%. Additionally, the predicted 3-year EFS rate for those in the pembrolizumab arm was 84.5%, while it was 76.8% for those in the placebo arm [8]. Interestingly, programmed death ligand-1 (PD-L1) expression was not adding benefit and currently pembrolizumab plus chemotherapy can be recommended without PD-L1 testing.
The presence of multiple primary tumors is rare in clinical practice. In our case, the occurrence of two highly malignant tumors and their treatment were described. In the diagnosis of synchronous tumors, the possibility of metastases from the second tumor must be excluded. The most common secondary cancers are breast carcinoma, sarcomas, and salivary gland neoplasms.
In the present case, a revision of histology was performed which proved the two simultaneous tumors. Sequencing the tumor genome gives us additional information. It is important to establish whether the tumors are hereditary or not. Sometimes, as we describe in this particular case, treatment for MPMNs can overlap and physicians should seek out for a drug or a combination of drugs that can treat both tumors at the same time in order to acquire better results.
For primary cutaneous melanoma following BC, the standardized incidence ratio (SIR) varied from 1.03 to 4.10, while for primary breast carcinoma following cutaneous melanoma, it varied from 1.16 to 5.13. Age, gender, and the use of chemotherapy and radiation therapy are all highlighted in epidemiological research as possible risk factors for second primary malignancies (SPMs). Any relationship with SPM including melanoma and BC may be partially explained by mutations in BRCA2, CDKN2A, CDK4, and BAP1 (Fig. 3).
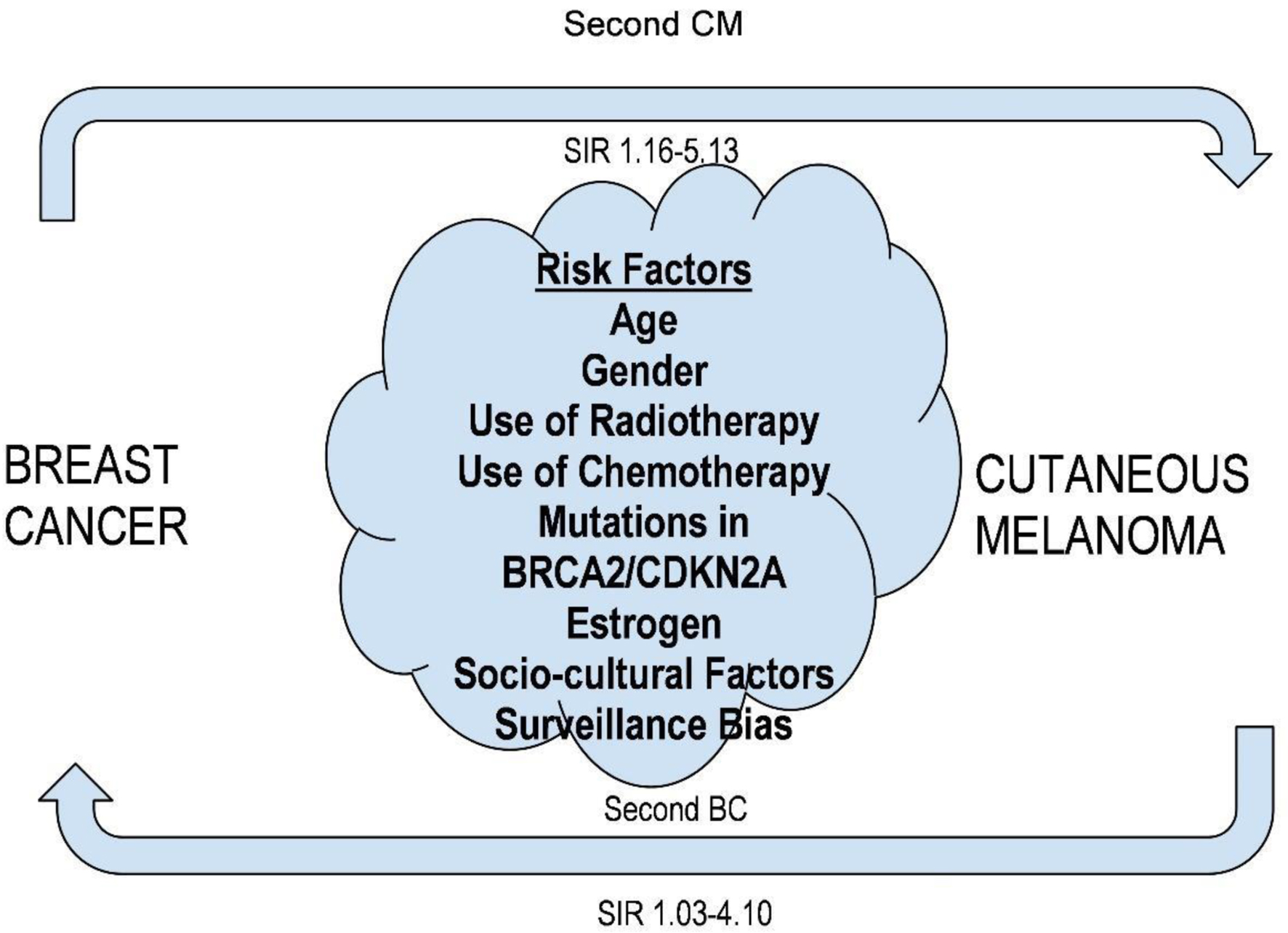 Click for large image | Figure 3. Risk factors of simultaneous occurrence of breast cancer and cutaneous melanoma. |
We identified articles, using PubMed, describing cases of primary cutaneous melanoma after BC or primary BC after cutaneous melanoma. There is strong evidence of association between BC and SPMs and according to some articles about a 17% increase in the risk of developing another primary neoplasm [13-15].
According to preclinical evidence, cancers that lack p16INK4a function might be susceptible to CDK4/6 inhibitors such palbociclib, abemaciclib, and ribociclib. Clinical evidence in uterine leiomyosarcoma, BC, and mesothelioma suggests that loss of CDKN2A may be a predictor of therapy sensitivity to abemaciclib and palbociclib. Nevertheless, p16INK4a loss or inactivation has not been significantly correlated with the therapeutic effect of these medicines in several other clinical investigations; hence, it is unknown if CDK4/6 inhibitors would be helpful in this situation [16-21].
Genomic profiling proves the link between the development of breast carcinoma and skin melanoma. This also provides an opportunity for further research and optimization of treatment for patients with these two tumors.
Learning points
Multiple primary tumors are rare and could be hard to treat. Several risk factors are associated with cancer formation and SPMs. Performing risk factors tests may be helpful for some patients with primary cancer, for the early detection of secondary cancer. BC is the most commonly diagnosed cancer in women. Cutaneous melanoma represents an aggressive tumor with a continuous increase in incidence. High-throughput technologies hold the promise of personalized therapy by enabling the use of genetic tests that can direct patient care choices. By determining when certain treatments are ineffective for certain individuals, such as chemotherapy, personalized medicine also seeks to avoid the use of potentially dangerous treatment modalities.
In this particular case, the treatment with immunotherapy-pembrolizumab and a combination of chemotherapy agents proved to be an effective treatment for both tumors and achieved a satisfactory outcome, intending to explore effective treatment modalities for this type of MPMNs, to improve the survival and prognosis of the patient.
Acknowledgments
None to declare.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was signed by the patient.
Author Contributions
Conceptualization: MT, YM; methodology: DM, RH; formal analysis: DM, RH; investigation: MT, YM; resources: YM, RH; data curation: YM; writing-original and draft preparation: MT, YM; writing-review and editing: AY; visualization: AY; supervision: AY. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Jeyakumar A, Chua TC, Lam AK, Gopalan V. The melanoma and breast cancer association: an overview of their 'second primary cancers' and the epidemiological, genetic and biological correlations. Crit Rev Oncol Hematol. 2020;152:102989.
doi pubmed - Swaroop VS, Winawer SJ, Kurtz RC, Lipkin M. Multiple primary malignant tumors. Gastroenterology. 1987;93(4):779-783.
doi pubmed - Parhizgar P, Bahadori Monfared A, Mohseny M, Keramatinia A, Hashemi Nazari SS, Rahman SA, Al Marzouqi A, et al. Risk of second primary cancer among breast cancer patients: A systematic review and meta-analysis. Front Oncol. 2022;12:1094136.
doi pubmed pmc - Powell S, Tarchand G, Rector T, Klein M. Synchronous and metachronous malignancies: analysis of the Minneapolis Veterans Affairs (VA) tumor registry. Cancer Causes Control. 2013;24(8):1565-1573.
doi pubmed - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(S3):43-46.
doi pubmed - Shah M, Osgood CL, Amatya AK, Fiero MH, Pierce WF, Nair A, Herz J, et al. FDA approval summary: pembrolizumab for neoadjuvant and adjuvant treatment of patients with high-risk early-stage triple-negative breast cancer. Clin Cancer Res. 2022;28(24):5249-5253.
doi pubmed - Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556-567.
doi pubmed - Luke JJ, Rutkowski P, Queirolo P, Del Vecchio M, Mackiewicz J, Chiarion-Sileni V, de la Cruz Merino L, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399(10336):1718-1729.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176-198.
doi pubmed pmc - Hallett RM, Dvorkin-Gheva A, Bane A, Hassell JA. A gene signature for predicting outcome in patients with basal-like breast cancer. Sci Rep. 2012;2:227.
doi pubmed pmc - Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, Minisini AM, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. 2014;19(6):608-615.
doi pubmed pmc - Logan JE, Mostofizadeh N, Desai AJ, Euw VONE, Conklin D, Konkankit V, Hamidi H, et al. PD-0332991, a potent and selective inhibitor of cyclin-dependent kinase 4/6, demonstrates inhibition of proliferation in renal cell carcinoma at nanomolar concentrations and molecular markers predict for sensitivity. Anticancer Res. 2013;33(8):2997-3004.
pubmed - Molina-Montes E, Pollan M, Payer T, Molina E, Davila-Arias C, Sanchez MJ. Risk of second primary cancer among women with breast cancer: a population-based study in Granada (Spain). Gynecol Oncol. 2013;130(2):340-345.
doi pubmed - Silverman BG, Lipshitz I, Keinan-Boker L. Second primary cancers after primary breast cancer diagnosis in Israeli women, 1992 to 2006. J Glob Oncol. 2017;3(2):135-142.
doi pubmed pmc - Molina-Montes E, Requena M, Sanchez-Cantalejo E, Fernandez MF, Arroyo-Morales M, Espin J, Arrebola JP, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;136(1):158-171.
doi pubmed - Elvin JA, Gay LM, Ort R, Shuluk J, Long J, Shelley L, Lee R, et al. Clinical benefit in response to palbociclib treatment in refractory uterine leiomyosarcomas with a common CDKN2A alteration. Oncologist. 2017;22(4):416-421.
doi pubmed pmc - Konecny G, Hendrickson A, Jatoi A, Burton J, Paroly J, Glaspy J, Dowdy S, et al. A multicenter open-label phase II study of the efficacy and safety of palbociclib a cyclin-dependent kinases 4 and 6 inhibitor in patients with recurrent ovarian cancer. J Clin Oncol. 2016;34:15(suppl):5557.
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
doi pubmed - Recalde M, Davila-Batista V, Diaz Y, Leitzmann M, Romieu I, Freisling H, Duarte-Salles T. Body mass index and waist circumference in relation to the risk of 26 types of cancer: a prospective cohort study of 3.5 million adults in Spain. BMC Med. 2021;19(1):10.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.








