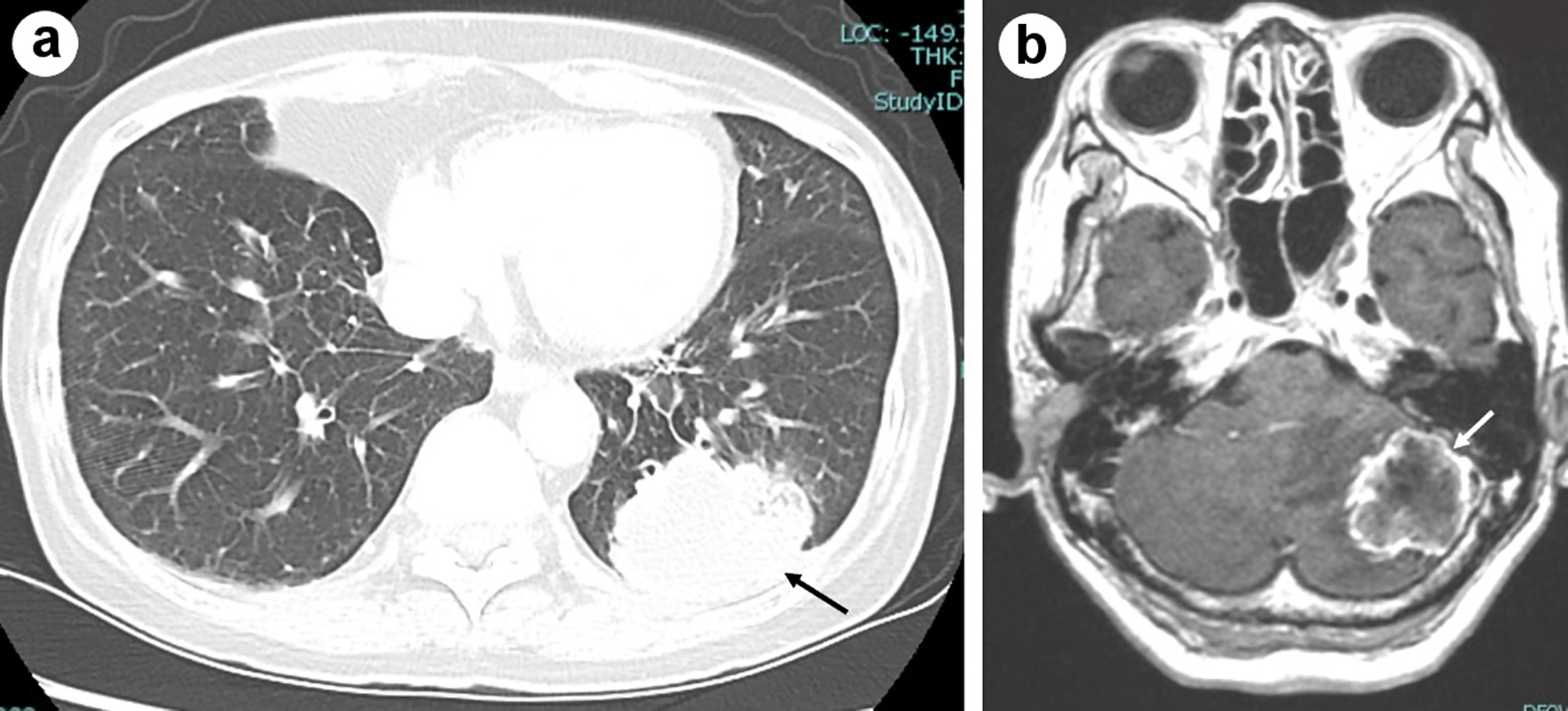
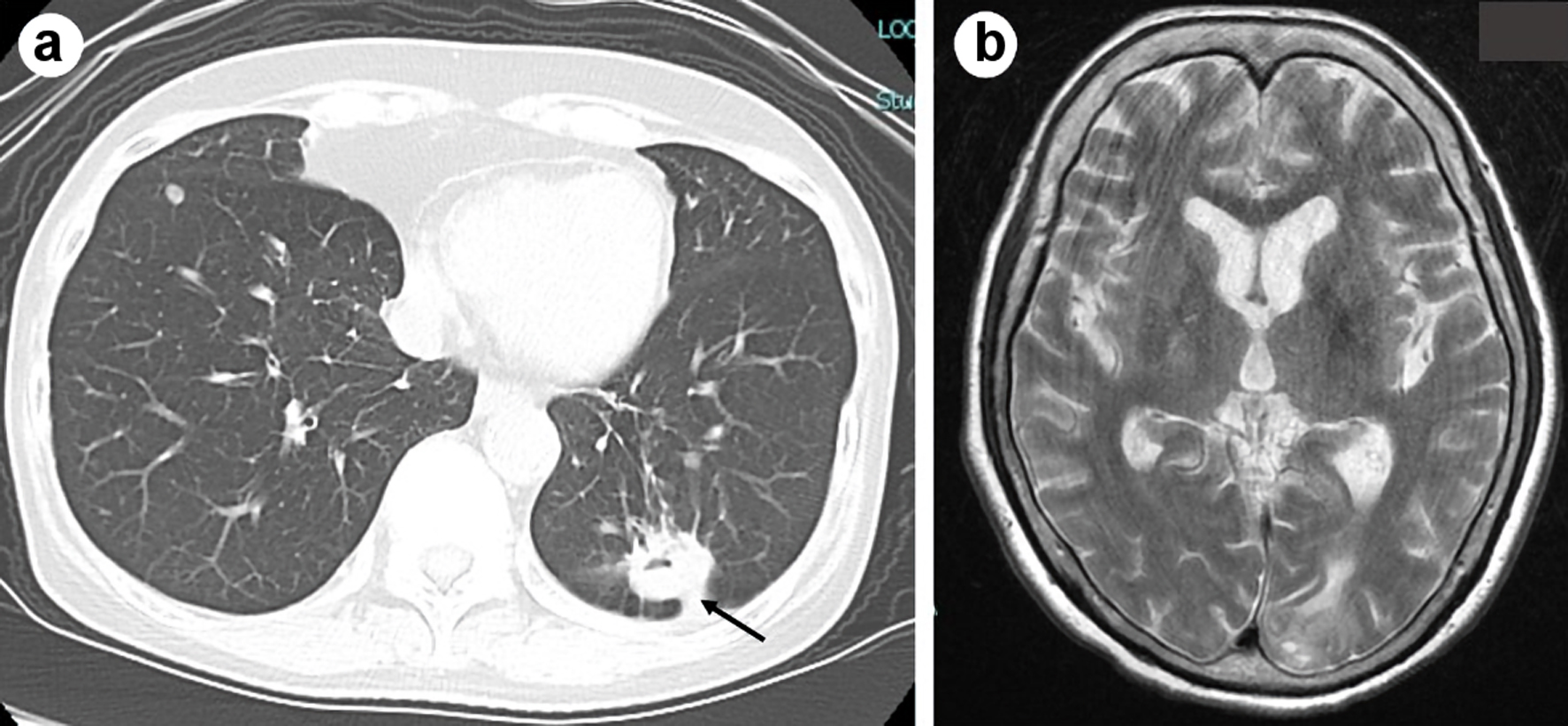
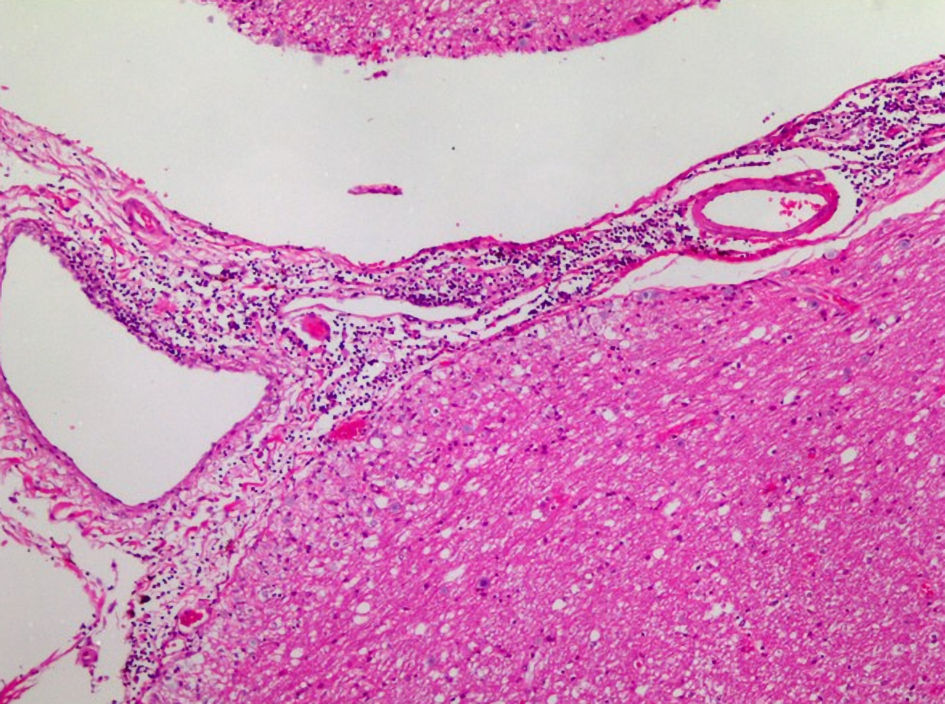
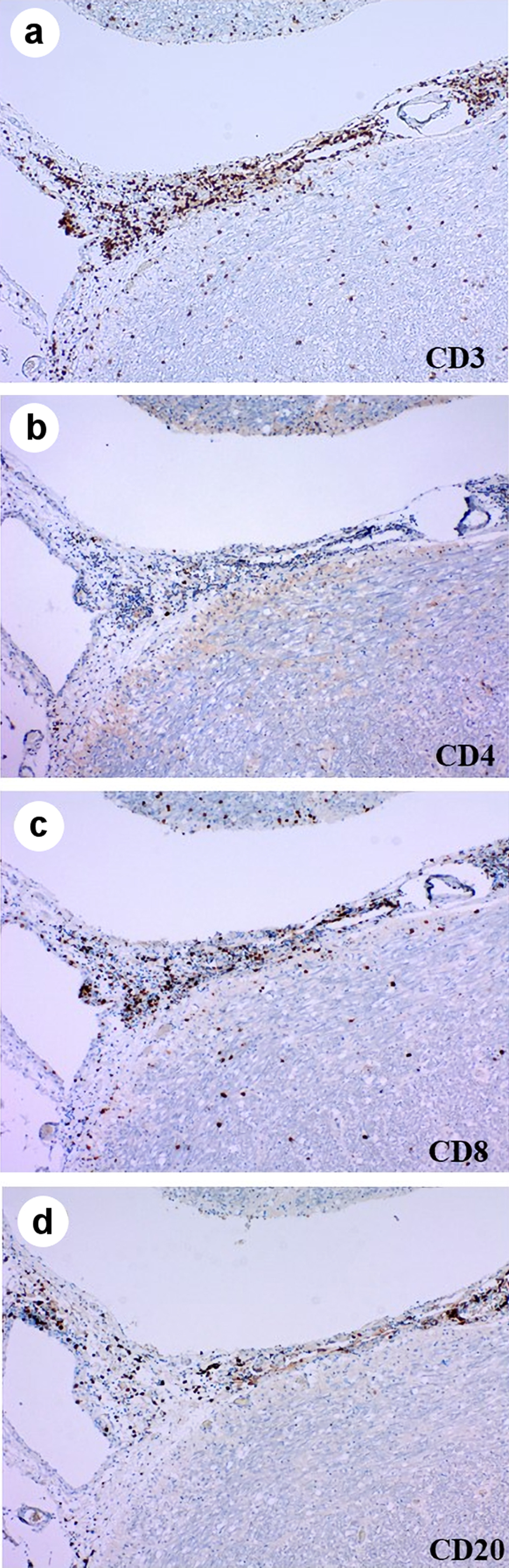
| Kawamura et al, 2016 [6] | 54/F | Ad | No | Nivo (1)/(ND) | > 4 weeks | ND | Dex, mPSL pulse | Negative | 56 mg/dL | 10/µL | Dead |
| Richard et al, 2017 [7] | 74/M | NSCLC | No | Nivo (1)/(ND) | 1 week | Yes | Dex | ND | ND | ND | Recovered |
| Raskin et al, 2017 [8] | 58/M | SQ | No | Nivo (17)/(PR) | 8 - 9 months | No | Dex | Hu | 74 mg/dL | 63/µL (96.8% mononuclear) | Recovered |
| Schneider et al, 2017 [9] | 78/F | SQ | No | Nivo (14)/(PR) | 28 weeks | No | mPSL | Negative | 1,027 mg/L | Lym 16/µL | Recovered |
| Leitinger et al, 2018 [4] | 67/F | SQ | No | Nivo (1)/(ND) | 17 days | No | mPSL, Ig, ACV | Negative | 56 mg/dL | LymMono 30/μL | Dead |
| Shah et al, 2018 [10] | 66/F | Ad | No | Nivo (ND)/(ND) | 4 months | No | mPSL pulse, PE | Novel and unclassified | 56 mg/dL | Normal | Clinically declined |
| 44/F | Ad | No | Nivo (5)/(ND) | 2.5 months | No | mPSL pulse, PE, rituximab | GAD65 | Normal | 19 nucleated cells/ (97% Lym) | Clinically declined |
| Matsuoka et al, 2018 [3] | 60/M | PC | No | Nivo (2)/(PR) | 33 days | No | High-dose mPSL, PE | Hu | 162 mg/dL | 16/µL | Dead |
| Niki et al, 2019 [11] | 51/M | SQ | Yes | Pem (ND)/(ND) | 8 months | No | High-dose mPSL | Negative | 446 mg/dL | 58/µL (Lym predominant) | Recovered |
| Honjo et al, 2019 [12] | 59/M | Large | Yes (mild) | Nivo (22)/(CR) | 16 months | No | mPSL pulse | Negative | 144 mg/dL | 61/µL (Mono 85%) | Recovered |
| Yonenobu et al, 2019 [13] | 61/M | SQ | Yes | Pem (2)/(ND) | 26 days | No | mPSL pulse, anti-TB, ACV | Negative | 209.2 mg/dL | 79/µL (Mono 100%) | Recovered |
| Fujiwara et al, 2019 [14] | 70/M | Ad | Yes (slight) | Nivo (ND)/(PR) | 5 months | No | High-dose mPSL, anti-TB, ACV | Negative | 166 mg/dL | 50/µL | Recovered |
| Arakawa et al, 2019 [15] | 78/M | Ad | Yes | Atezo (1)/(ND) | 13 days | Yes | mPSL pulse, ACV | Negative | 106 mg/dL | 6/µL | Recovered |
| Yamaguchi et al, 2020 [16] | 56/M | Ad | Yes | Atezo (1)/(ND) | 17 days | No | mPSL pulse | Negative | 166 mg/dl | 20/µL | Recovered |
| Robert et al, 2020 [17] | 48/F | Ad | Yes | Atezo (1)/(ND) | 13 days | No | mPSL pulse | ND | ND | 62 nucleated cells/µL (Neu predominant) | Recovered |
| 57/M | Ad | No | Pem (3)/(ND) | 4 months | No | mPSL pulse | ND | 1.41 g/L | 40 nucleated cells/µL (Lym 98%) | Recovered |
| Singh et al, 2021 [18] | 72/F | Ad | Yes | Ipi + Nivo (4)/(PD) | 13 weeks | Yes | High-dose mPSL | Negative | > 180 mg/dL | ND (Lym predominant) | Recovered |
| Ours | 67/F | Ad | Yes | Pem (13)/(PR) | 8 months | Yes | mPSL pulse, ACV | ND | 317.6 mg/L | 197 cells/µL (Mono 93%) | Dead |