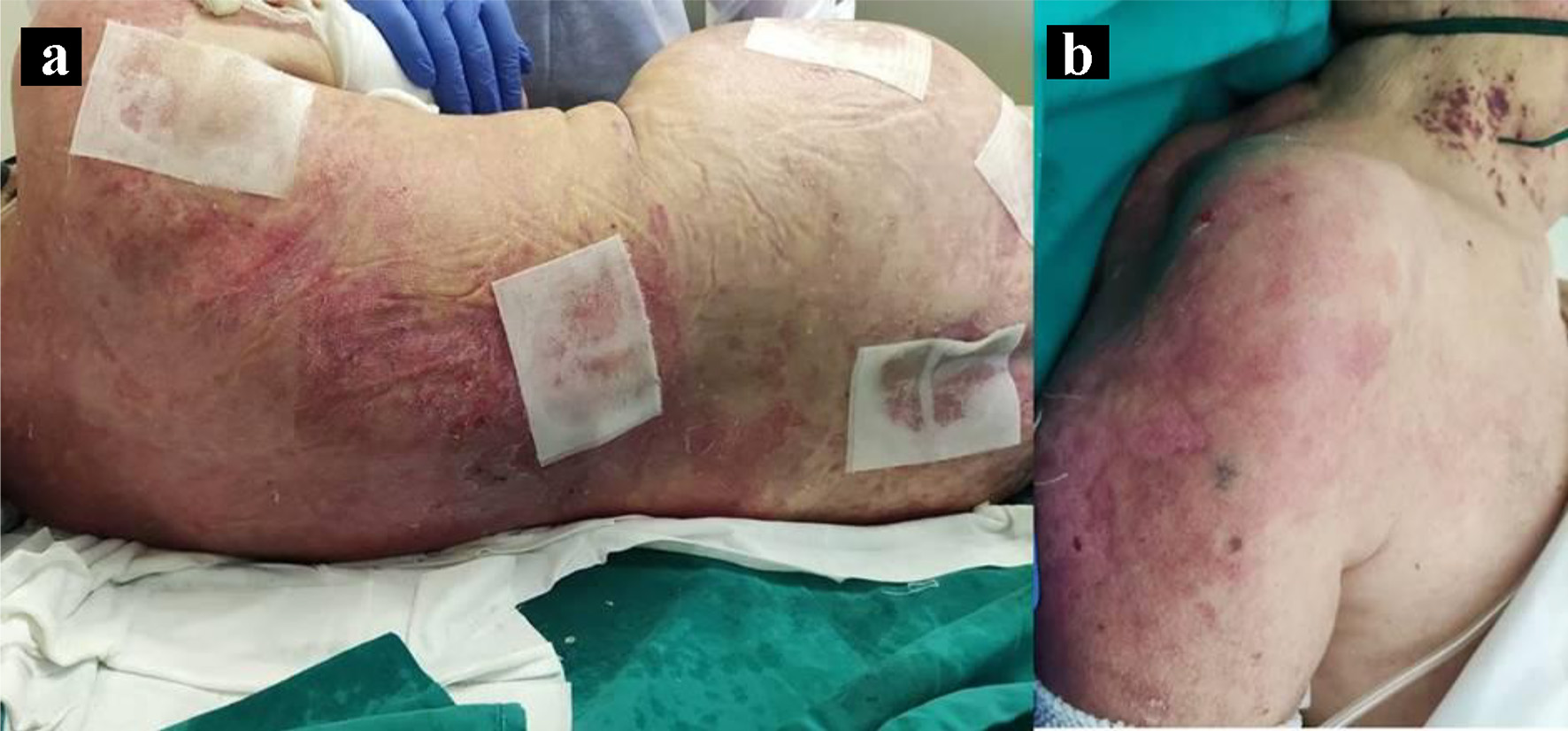
Figure 1. Toxic epidermal necrolysis (TEN)-like lesions in subacute cutaneous lupus (SCL). Large sheets of detached epidermis along the patient’s dorsum (a) and left shoulder (b).
| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 2, February 2022, pages 89-93
Toxic Epidermal Necrolysis-Like Lupus Erythematous Presentation Following SARS-CoV-2 Infection
Figures

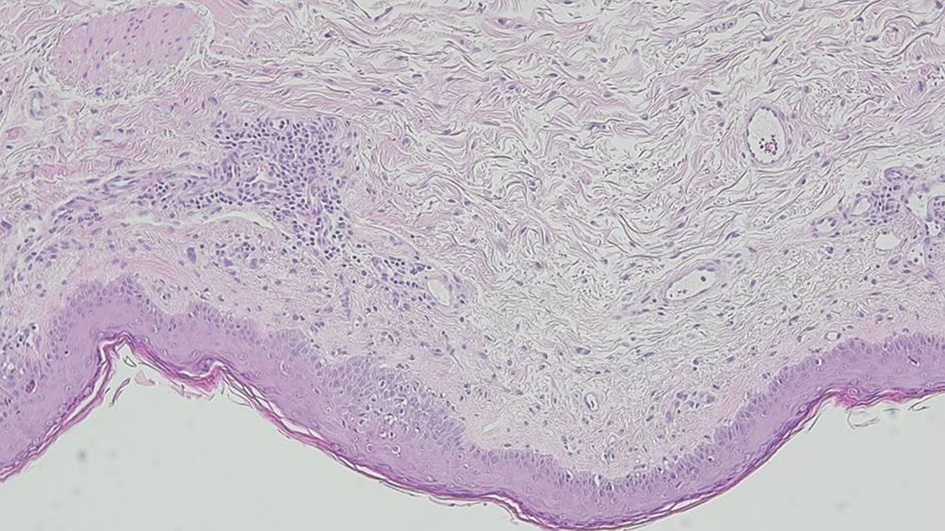
Tables
| Drug | ALDEN score | ||||||
|---|---|---|---|---|---|---|---|
| Causal link | Total ALDEN score | Type of drug | Dechallenge | Prechallenge/rechallenge | Drug present in the body on index day | Delay from initial drug component intake to onset of reaction | |
| Paracetamol | Very unlikely | -2 | Associated: 2 Drug with definite but lower risk | Negative: -2 Drug continued without harm | Negative: -2 Exposure without reaction | Excluded: -3 Drug stopped | Suggestive: +3 From 5 to 28 days |
| Sodium enoxaparin | Very unlikely | -5 | Not suspected: -1 No evidence of association | Negative: -2 Drug continued without harm | Negative: -2 Exposure without reaction | Excluded: -3 Drug stopped | Suggestive: +3 From 5 to 28 days |
| Ipratropium bromide | Very unlikely | -3 | Not suspected: -1 No evidence of association | Neutral: 0 Drug stopped or unknown | Negative: -2 Exposure without reaction | Excluded: -3 Drug stopped | Suggestive: +3 From 5 to 28 days |
| Dexamethasone | Very unlikely | -1 | Not suspected: -1 No evidence of association | Neutral: 0 Drug stopped or unknown | Not done/unknown: 0 No known previous exposure | Excluded: -3 Drug stopped | Suggestive: +3 From 5 to 28 days |
| Telmisartan | Very unlikely | -3 | Unknown: 0 | Neutral: 0 Drug stopped (or unknown) | Negative: -2 Exposure without reaction | Definite: 0 Drug continued | Unlikely: -1 > 56 days |
| Result (normal range) | |
|---|---|
| IgG: immunoglobulin G; IgM: immunoglobulin M; EBNA: Epstein-Barr nuclear antigen; HBsAg: hepatitis B surface antigen; HBsAb: hepatitis B surface antibody; HBcAb: hepatitis B core antibody; Ag: antigen; Ab: antibody. | |
| Blood test | |
| Leucocytes | 14.3 × 109/L (3.6 - 10.5 × 109/L) |
| Neutrophils | 9.3 × 109/L (1.5 - 7.7 × 109/L) |
| Hemoglobin | 9.9 g/dL (12 - 16 g/dL) |
| Mean corpuscular volume | 84 fL (80 - 100 fL) |
| Erythrocyte sedimentation rate | 51 mm/h (1 - 20 mm/h) |
| C-reactive protein | 20.1 mg/dL (0.5 - 1 mg/dL) |
| Procalcitonin | 0.67 ng/mL (0 - 0.5 ng/mL) |
| Lactate dehydrogenase | 585 U/L (< 247 U/L) |
| Ferritin | 2,790 ng/mL (30 - 300 ng/mL) |
| Albumin | 1.6 g/dL (3.5 - 5.2 g/dL) |
| Immune and autoimmune tests | |
| Complement component 3 | 0.66 g/L (0.83 - 1.93 g/L) |
| Complement component 4 | 0.1 (0.15 - 0.57) |
| Antinuclear antibodies | Positive (1:1,280, nuclear homogeneous pattern) |
| Extractable nuclear antigens antibodies | SSA60 (Ro60)/SSB (La) positive |
| Urine analysis | |
| 24 h proteinuria | 642 mg/24 h (50 - 80 mg/24 h) |
| Blood serologies | |
| Cytomegalovirus | Negative (IgM and IgG) |
| Herpes simplex virus 1 and 2 | Negative (IgM and IgG) |
| Epstein-Barr virus | Negative (IgM, IgG and EBNA) |
| Hepatitis B virus | Negative (HBsAg, HBsAb and HBcAb) |
| Hepatitis C virus | Negative (IgM and IgG) |
| Hepatitis A virus | Negative (IgM and IgG) |
| Human immunodeficiency virus | Negative (Ag/Ab) |
| Treponema pallidum | Negative (IgM and IgG) |
| Rickettsia conorii | Negative (IgM and IgG) |
| Coxiella burnetii | Negative (IgM and IgG) |