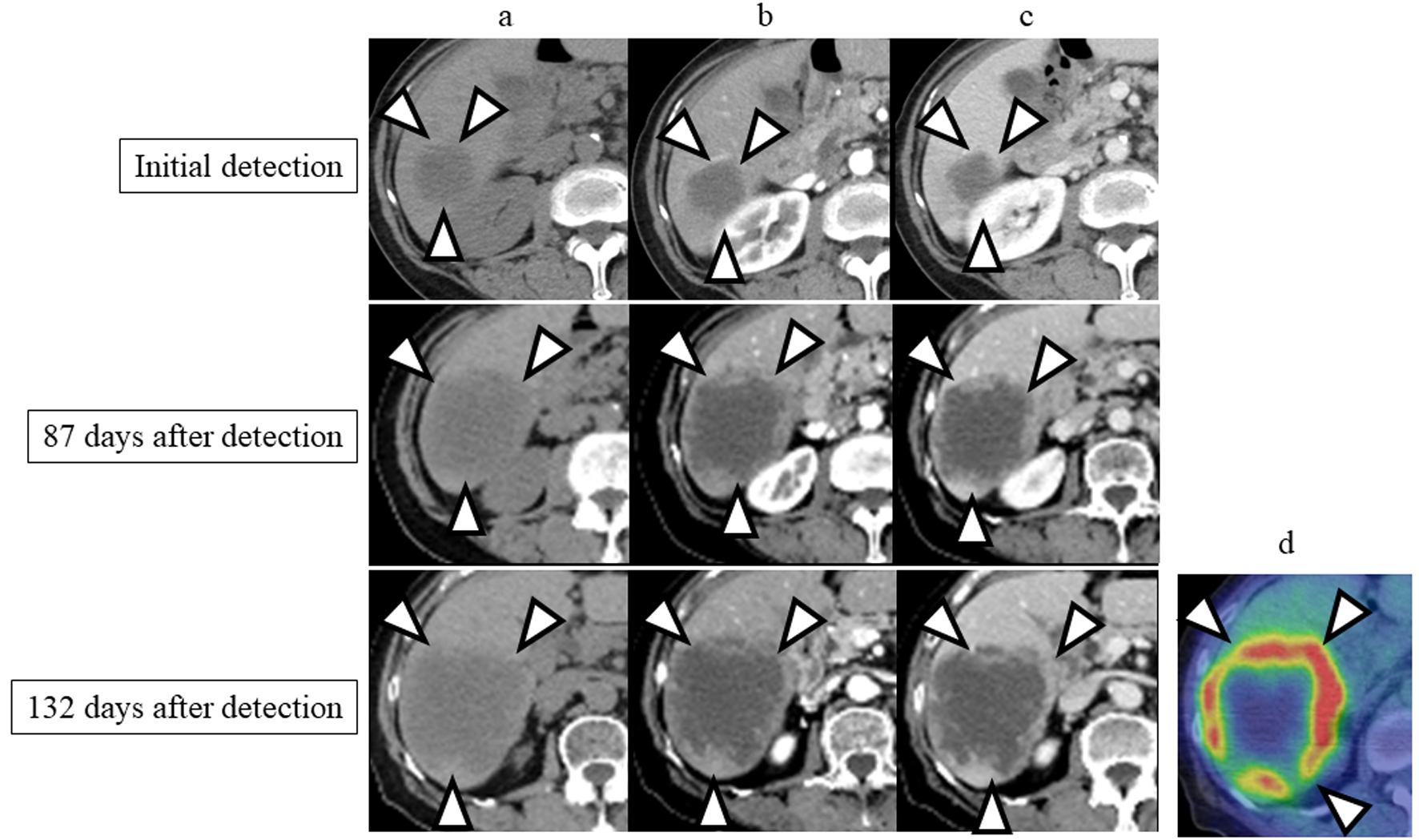
Figure 1. Enhanced computed tomography (CT) images performed at the time of detection and 87 and 132 days after the initial detection. (a) Precontrast phase; (b) arterial phase; (c) portal phase; (d) 18F-fluorodeoxyglucose positron emission tomography (PET)/CT performed at 139 days after the initial detection. The tumor is indicated by arrowheads.
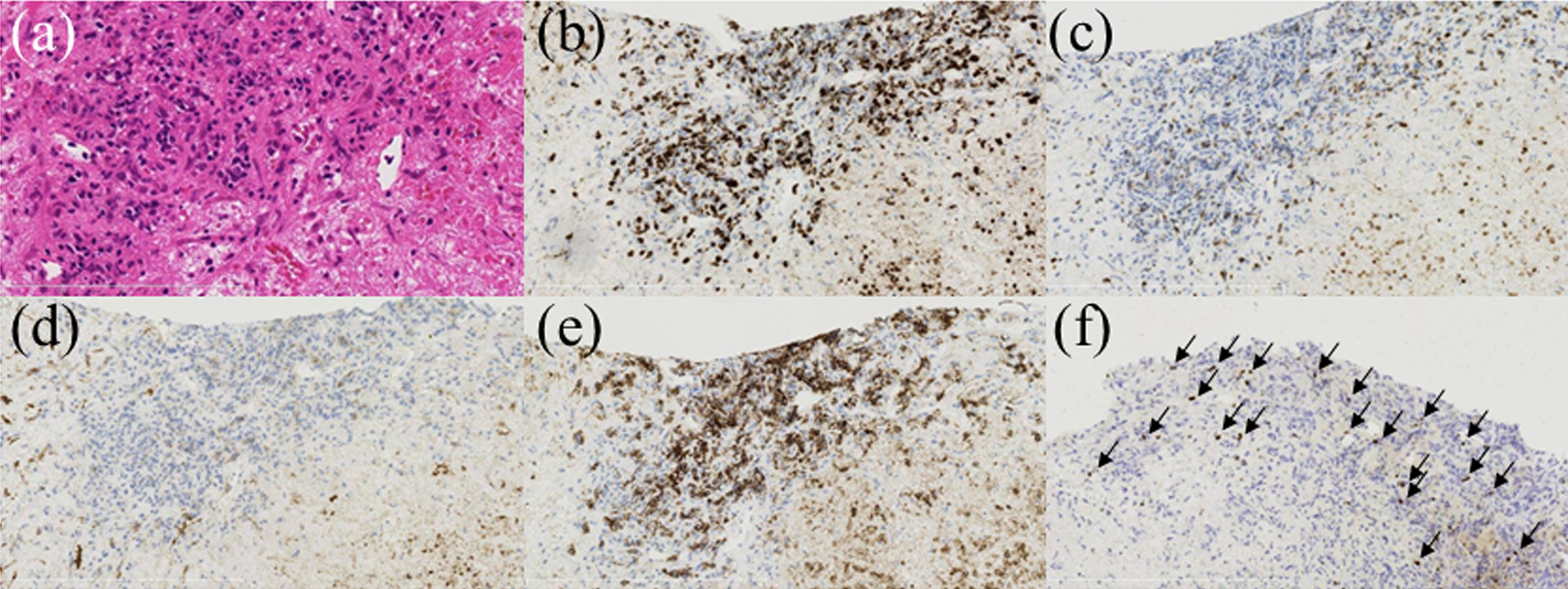
Figure 2. Pathological findings of the liver biopsy. Hematoxylin and eosin (H&E) staining revealed lymphocyte proliferation and a wide variety of forms. The tumor cells were positive for CD20 and CD3 and negative for CD5 and CD10. In situ hybridization revealed partial positivity for EBV-encoded small RNAs (EBERs). (a) H&E staining, × 400; (b) anti-CD3 staining, × 100; (c) anti-CD5 staining, × 100; (d) anti-CD10 staining, × 100; (e) anti-CD20 staining, × 100; (f) in situ hybridization EBERs, × 100 (arrows denote cells positive for EBERs on in situ hybridization).

