| 1 | October 8, 2020 | LALA-94 protocol + imatinib 400 mg daily (performed at outside hospital) | None | 0.14% | NA |
| 2 | December 9, 2020 | Chemo + dasatinib 140 mg daily | None | 19.4% | 5.3121% |
| 3 | January 19, 2021 | Blinatumomab (clinical trial), January 11, 2021 | None | NA | Negative, 0.001, 0.0008 |
| 4 | September 22, 2021 | Blinatumomab + nilotinib | None | Negative | 0.012 |
| 5 | October 27, 2021 | Dose reduced - hyperCVAD-part B + nilotinib
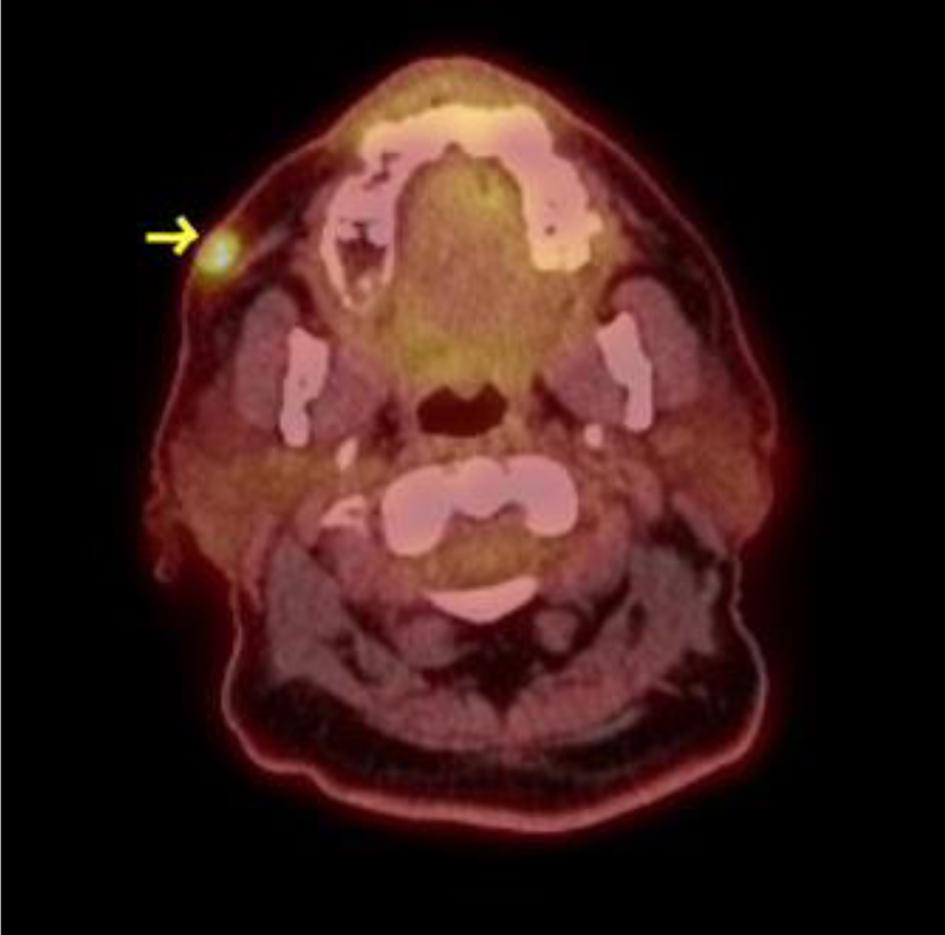
D1: MTX 100 mg/m2 (2 h), 400 mg/m2 over 22 h; D2-3 cytarabine 1 g/m2 q 12 h | Right cheek mass
Abdominal nodal mass | Negative | Negative |
| 6 | February 8, 2022 | Ponatinib single agent | None | NA | Negative |
| 7 | March 8, 2022 | Ponatinib 15 mg daily, vincristine 1 mg IV and prednisone 60 mg day 1 - 5 Q 4 weeks | None | NA | Negative, 4.2% |
| 8 | December 16, 2022 | Repeat reduced dose hyper-CVD-part B
Methotrexate 100 mg/m2 for 2 h then 400 mg/m2 for 22 h
Cytarabine 500 mg/m2 q12 h for 2 days
IT chemotherapy 70 mg cytarabine day 2 and 12 mg methotrexate on day 8
Started ponatinib 15 mg daily from day 5
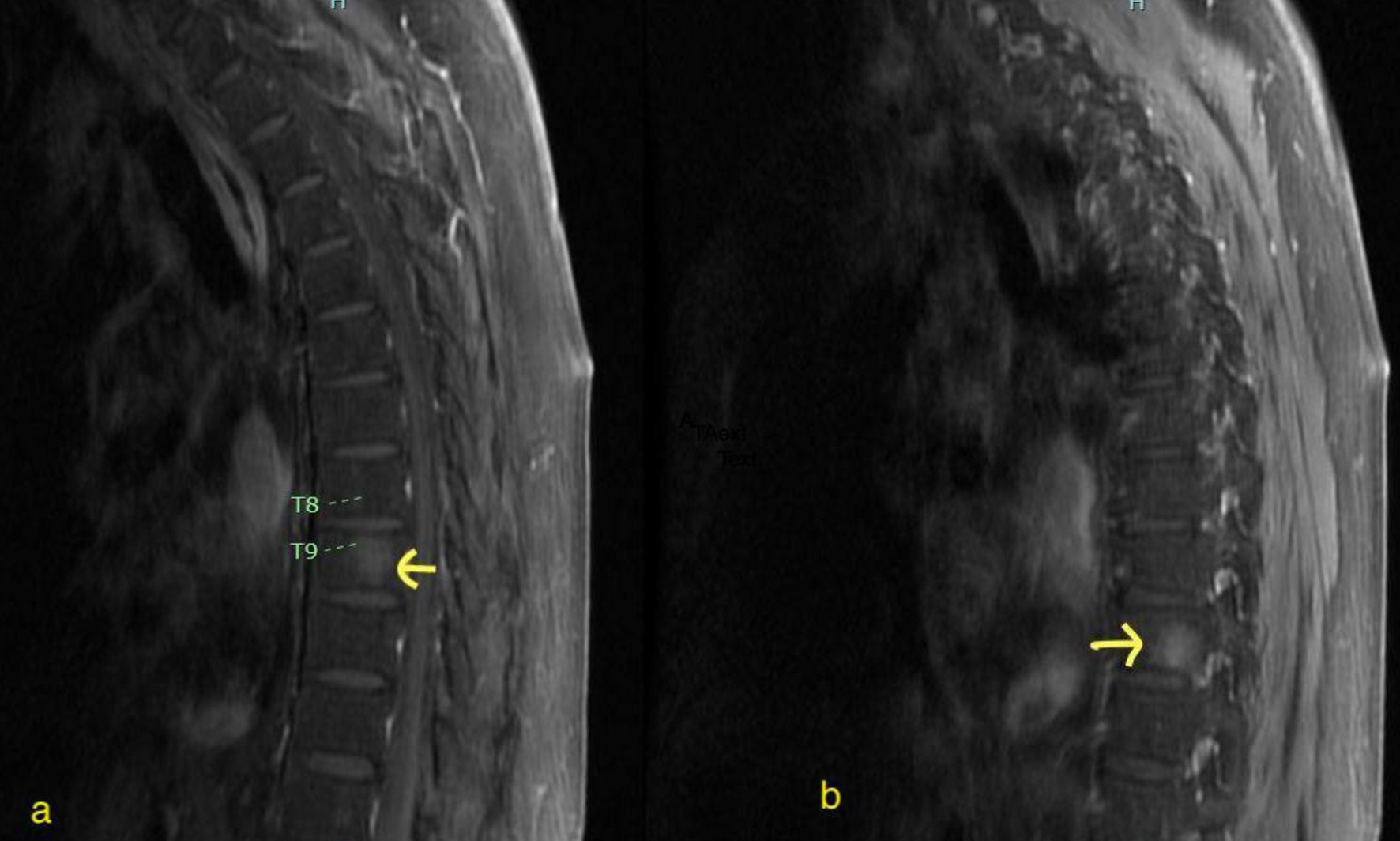
Increased dose to 30 mg daily from day 10 | PET scan showed extramedullary disease- involving T9 vertebral body, fourth rib and pelvic lymphadenopathy
CNS disease detected | 0.047 | 0.395 (ABL kinase mutation negative) |
| 9 | January 25, 2023 | Ponatinib 30 mg daily with asciminib 40 mg twice a day (maintenance prior to brexu-cel) | Vertebral bone disease | NA | 0.0071 |
| 10 | March 20, 2023 | Brexucabtagene-autoleucel | Bone lesions involving thoracic and vertebra, resolution of pelvic lymphadenopathy | Negative | Negative on day 60 bone marrow biopsy/PET scan negative at day 100. |
| 11 | April 6, 2023 | Resumed ponatinib 15 mg daily with asciminib 40 mg twice a day (maintenance post brexu-cel) | None | 9.3 | 12.96 (ABL1 kinase mutation negative) |
| 12 | February 22, 2024 | Repeat course of blinatumomab (later nilotinib 200 mg twice a day was added) | None | Negative (May 14, 2024) | Negative (July 17, 2024) |