| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 9-10, October 2023, pages 332-338
Synchronous Left Ventricular and Endocranial Mass
Cornelia Tsokkoua, Andreas Mitsisa, d, Evi Christodouloub, Panayiotis Avraamidesa, Stefanos Sakellaropoulosc
aCardiology Department, Nicosia General Hospital, Nicosia, Cyprus
bCardiology Department, Limassol General Hospital, Limassol, Cyprus
cDepartment of Internal Medicine, Cardiology Clinic, Kantonsspital Baden, Baden, Switzerland
dCorresponding Author: Andreas Mitsis, Cardiology Department, Nicosia General Hospital, Nicosia, Cyprus
Manuscript submitted September 1, 2023, accepted October 6, 2023, published online October 13, 2023
Short title: Challenges in the Diagnosis of Cysticercosis
doi: https://doi.org/10.14740/jmc4153
| Abstract | ▴Top |
Myocardial cysts represent a miscellaneous and infrequent spectrum of conditions, with each of them coming from a different etiological background. Congenital myocardial cysts, neoplasia, cysts of infectious origin (bacterial, viral, or parasitic), and cardiac pathologies that may fake cystic content are all encompassed in this group. Although most patients are asymptomatic, some may occasionally present with obstruction, valvular dysfunction, or heart failure. Even more uncommon is the coexistence of a myocardial cyst with other extracardiac locations causing extracardiac symptoms. In this direction, the coexistence of a myocardial and endocranial cyst is extremely rare and can cause symptomatology from the affected organs (e.g., seizures). Cardiac investigation in this context is mainly dependent on non-invasive diagnostic modalities, and laboratory procedures. In this case report, we present a 26-year-old Congolese male admitted with dyspnea and epileptic seizures. Echocardiography revealed left ventricular and both mitral and tricuspid valve dysfunction and the presence of two myocardial cysts, while brain computed tomography showed an additional frontal cystic lesion. A precise diagnostic workup with a combination of non-invasive imaging, laboratory results, and epidemiology data assisted the diagnosis and guided the most suitable therapeutic choice.
Keywords: Endocranial cyst; Heart failure; Myocardial cyst; Epileptic seizures; Parasitical diseases; Cysticercosis
| Introduction | ▴Top |
A myocardial cyst is a rare manifestation of conditions such as endocarditis, bacteremia, or other miscellaneous causes including thoracic trauma and/or burn, suppurative pericarditis and parasitical diseases [1]. Due to the hidden nature of myocardial cysts, the patients usually remain asymptomatic until lesions reach a proper size to cause specific cardiovascular symptoms. The minority of patients with larger myocardial cysts can present with precordial chest pain, arrhythmias, and dyspnea, which can make it difficult to differentiate it from other cardiac entities. Even more rarely, synchronous extracardiac cysts are present, but again the diagnosis is delayed unless the size and location of the cysts cause specific signs and symptoms.
The diagnosis of myocardial cysts relies on multimodality imaging, including echocardiography, computed tomography (CT) scan, and magnetic resonance imaging (MRI). Furthermore, laboratory tests, and epidemiology data are paramount to confirm the diagnosis. Treatment of a cardiac cyst is usually surgical. Surgical exclusion of the cyst, apart from therapeutic can add useful diagnostic information. Excisional biopsy provides useful histopathological information and most of the times assist the diagnosis and guide the most therapeutic choices.
We present an unusual case of a patient with a large myocardial cyst with abscess features which was associated with a severely impaired left ventricular (LV) dysfunction, and a synchronous endocranial mass manifested as epileptic seizures.
| Case Report | ▴Top |
Investigations
A 26-year-old Congolese male was transferred from a refugee camp to the emergency department of our hospital due to severe dyspnea, orthopnea, and generalized peripheral edema. His past medical history was unremarkable. Upon clinical examination, a holosystolic murmur was auscultated at the heart apex and bilateral rales were present on lung auscultation. He was hemodynamically stable with a blood pressure of 125/80 mm Hg and a heart rate of 100 bpm. Laboratory tests were mostly unremarkable, except for a D-dimer of 1,303 ng/mL and an elevated C-reactive protein (CRP) of 58.6 mg/L. The 12-lead electrocardiography (ECG) showed sinus tachycardia at 102 bpm with LV hypertrophy and LV strain and the chest X-ray revealed mild lung congestion.
Diagnosis
While in the emergency department, the patient developed epileptic seizures and progressed to status epilepticus, leading to patient’s intubation, and was placed on mechanical ventilation for airway protection. A brain CT was done in an emergency basis showing a cystic lesion with a diameter of 14 mm, in the right frontal lobe (Fig. 1a). This finding was afterwards confirmed by a brain MRI (Fig. 1b, c). Anti-epileptic treatment with levetiracetam was started.
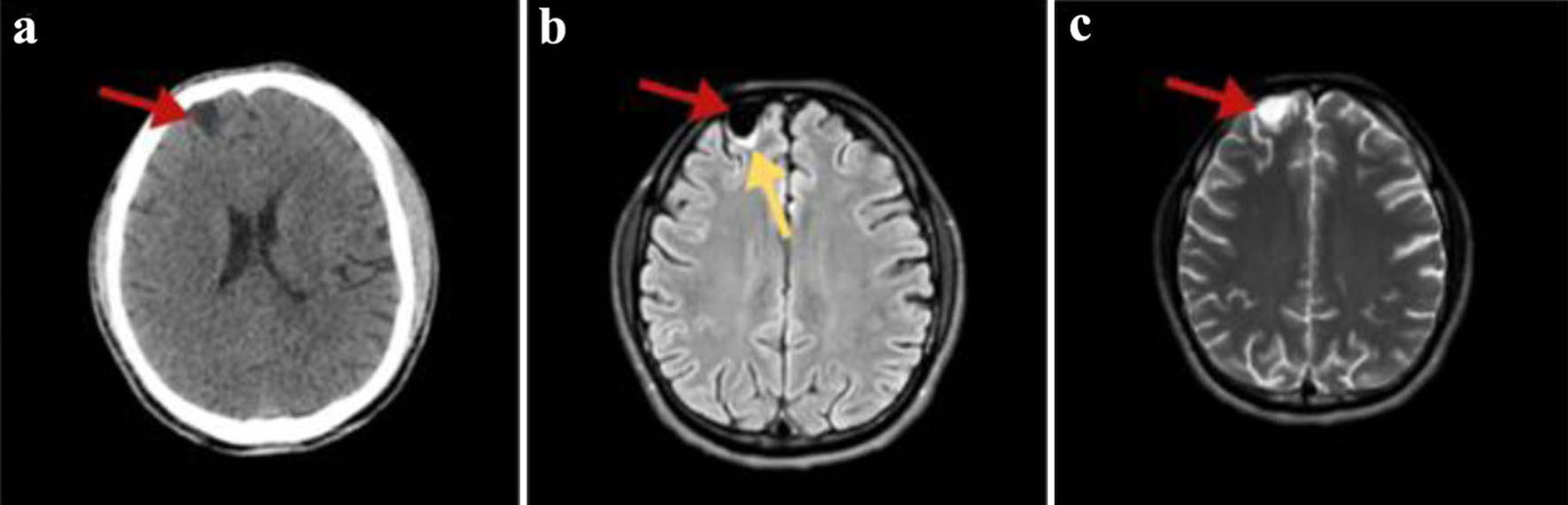 Click for large image | Figure 1. (a) Non-enhanced axial brain computed tomography showing a hypodense right frontal cystic lesion (red arrow). (b) FLAIR MRI axial slices depicting a hypointense homogenous frontal cystic lesion (red arrow) with surrounding gliotic changes (yellow arrow). (c) T2-weighted brain MRI image in the transverse plane presenting the lesion having a non-homogenous pattern (red arrow). In all MRI images, the cyst has similar signal and intensity to those of CSF. MRI: magnetic resonance imaging; FLAIR: fluid-attenuated inversion recovery; CSF: cerebrospinal fluid. |
Transthoracic echocardiography (TTE) showed biventricular dilatation and a severely impaired LV systolic function with a left ventricular ejection fraction (LVEF) of 20-25%. A large interventricular cyst measured 5.8 × 4.6 cm was identified (Fig. 2a, b). Furthermore, moderate mitral regurgitation (MR), and severe tricuspid regurgitation (TR) with right ventricular systolic pressure (RVSP) of 60 mm Hg and signs of pressure and volume overload were also shown (Fig. 2a, b). A transesophageal echocardiogram (TEE) confirmed the presence of the interventricular cyst and unmasked a communication of the cyst with the left ventricle. In addition, a smaller paravalvular cystic lesion was revealed next to the anterior leaflet (A1 - anterior segment) of the mitral valve (measured 1.9 × 2.4 cm). Both above cysts had hypoechogenic content with hyperechogenic walls (Fig. 2c, d). Cardiac MRI was performed a few days later confirming the previous findings (Fig. 3a-c).
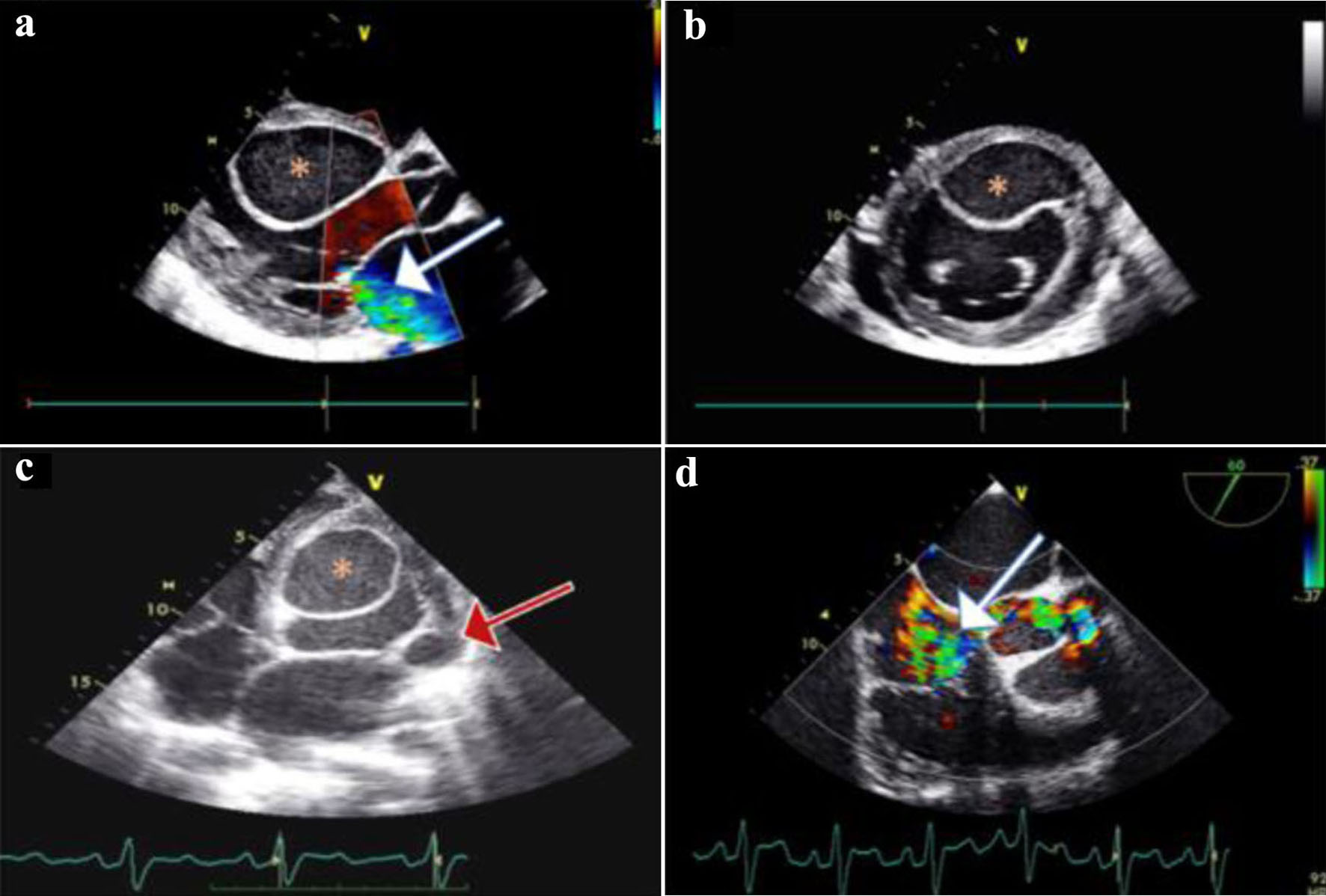 Click for large image | Figure 2. (a) Two-dimensional transthoracic echocardiography (TTE) of parasternal long-axis, showing mild to moderate mitral regurgitation (MR) (white arrow); a large cyst (asterisk) is noticed on the interventricular septum (IVS) protruding in the left ventricle (LV). (b) TTE of parasternal short-axis view showing the IVS cyst protruding in the LV cavity; a small pericardial effusion is also inspected. (c) Transesophageal echocardiogram (TEE) of four-chamber view showing the IVS cyst, and a smaller cyst (red arrow) on the lateral annulus of the mitral valve. (d) TEE mid-esophageal inflow - outflow 60° view showing severe tricuspid regurgitation (TR) (white arrow). |
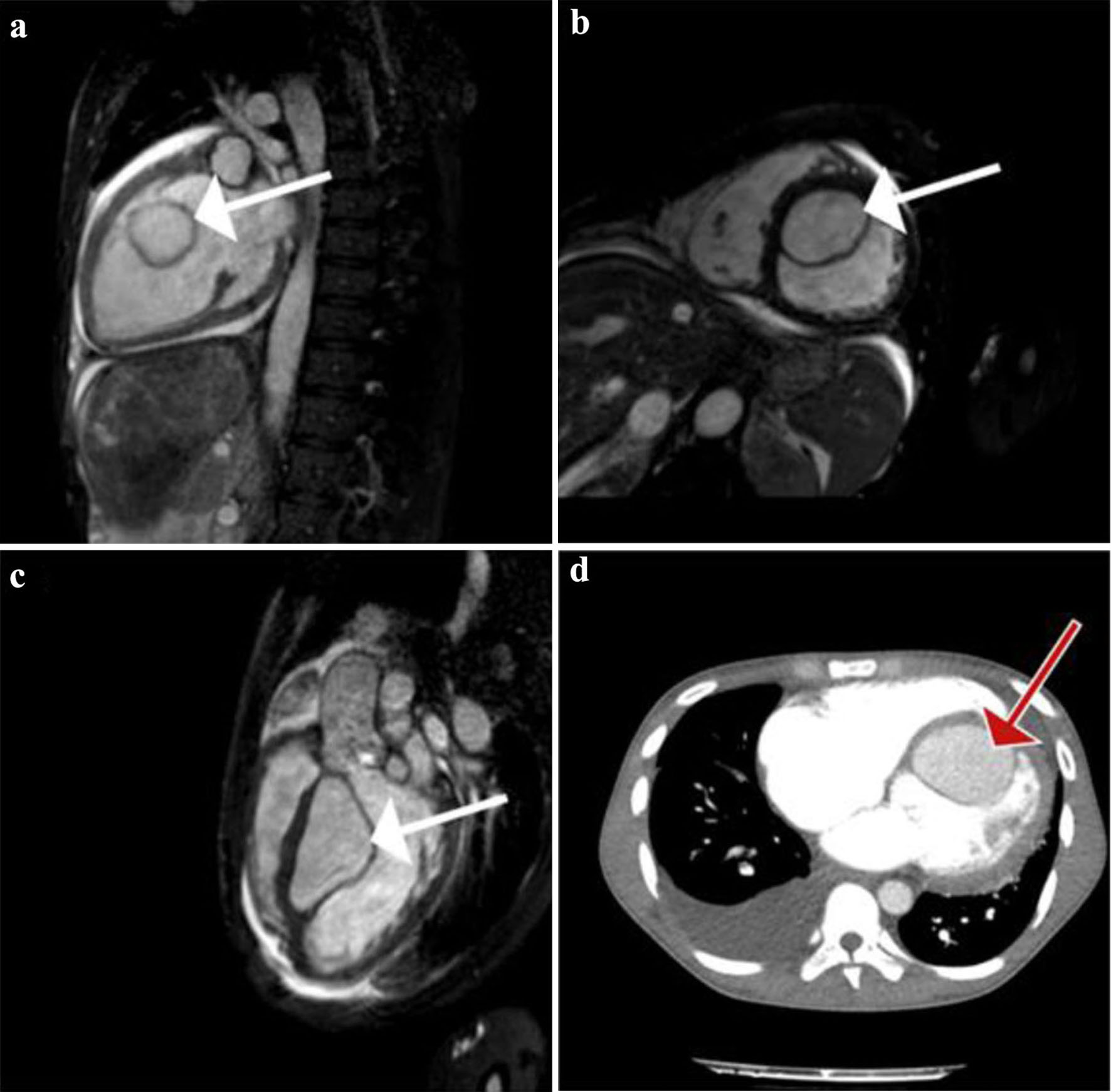 Click for large image | Figure 3. (a) Gadolinium-enhanced vertical long-axis cardiovascular magnetic resonance (CMR) view (or pseudo two-chamber view) clearly showing a large left ventricular (LV) cyst (white arrow). Homogenous enhancement of the cyst and LV’s content implicate the existence of a communication between the two. (b) True short-axis CMR view, where the cyst (white arrow) is seen attached to the interventricular septum (IVS). Simultaneously we see the dilated right ventricular (RV) cavity. (c) Sagittal left ventricular outflow tract obstruction (LVOT) CMR view showing again the intraventricular cyst (white arrow) attached to the IVS. (d) Transverse contrast-enhanced computed tomography (CT) pulmonary angiogram, depicting a large right pleural effusion and the large LV cyst (red arrow). No signs of pulmonary embolism were seen. |
The patient was supported with inotropes, vasopressors as well as intravenous diuretics. His condition improved gradually, and he managed to be extubated successfully a few days after his admission. Serological laboratory tests did not reveal any specific recent infection, i.e., bacterial, viral (including human immunodeficiency virus (HIV) infection), or parasitical (including echinococcus and toxoplasma gondii antibodies) and blood cultures were all negative. In view of possible parasitic infectious disease, albendazole 400 mg b.i.d for a period of 6 weeks was also started.
Concluding our investigation, a thorax abdominal CT scan was done which did not reveal any new cystic lesions apart from the large endocardial interventricular cyst (Fig. 3d). Finally, a computed tomography pulmonary angiogram (CT-PA) excluded pulmonary embolism, exposing only a small right pleural effusion and an opacification with air bronchogram in the right middle lobe.
Treatment
We decided to refer the patient for exclusion of the cardiac cyst and repair of the tricuspid valve. The procedure was performed successfully, and the cyst was surgically removed. Histopathologic examination of the cyst divulged pieces of flat fibrous tissue devoid of epithelial or endocardial lining. Overlying the fibrous tissue, there was a prominent fibrin layer and a mixed adjacent inflammatory cell infiltrate, suggesting chronic inflammation. No granulomas or evidence of malignancy were seen. The diagnosis of inflammatory cysts keeping up with abscess’ features was made. Biopsy’s tissue culture was without any growth, and microscopy did not reveal anything of importance.
Follow-up and outcomes
After a short post-operative period, the patient was discharged on optimal heart failure therapy including maximum tolerated doses of angiotensin-converting enzyme inhibitor (ACE-I) (ramipril 2.5 mg/day), beta-blockers (bisoprolol 1.25 mg/day), loop diuretics (furosemide 40 mg/day), aldosterone receptor antagonists (spironolactone 50 mg/day), sodium-glucose transport protein 2 (SGLT2) inhibitors (dapagliflozin 10 mg/day), and on a combination of antiepileptic agents (levetiracetam and valproic acid) and antiparasitic with albendazole 400 mg b.i.d for a period of 6 weeks. After 12 months, he remains stable and symptom-free with no exacerbation of his heart failure or further seizures. The follow-up brain CT was repeated after a 6-month period and did not show any growth or change of the cyst.
| Discussion | ▴Top |
To our knowledge, this is the first case in the literature of a patient with concomitant myocardial and endocranial cyst with a synchronous presentation of this clinical symptomatology. A comprehensive evaluation of cyst morphology and tissue composition together with all available imaging modalities including CT and MRI, in conjunction with the epidemiological status, and the available laboratory results, guided our attentiveness mainly towards parasitic infections and especially cysticercosis which was the disease more compatible with this case’s presentation.
Intra-cardiac cysts represent a diverse and an exceedingly infrequent spectrum of conditions, encompassing congenital pathologies such as blood cysts or foregut cysts, and neoplasia including teratomas and myxomas. Other important entities in this spectrum are parasitic cysts which are expounded later in detail and cardiac conditions which can occasionally feign cystic content, such as intracardiac thrombi. Blood cysts are usually congenital abnormalities that are encountered in infants before the age of 6 months, and that resolve before adulthood. Mitral valve is the predominant site of attachment. Rarely they could be found attached in the cardiac chambers. On the other hand, blood cysts may acquire after cardiothoracic surgery, blunt trauma, or inflammatory process [1]. A foregut cyst is the result of anomalous embryonic development and contains endodermal and mesodermal elements. Thus, a foregut cyst is a generic name which encompasses enteric, esophageal, gastric, and bronchogenic cysts, a subdivision based on histological examination. From the subdivisions, enteric and bronchogenic cardiac cysts have been described across the years, while the rest could be found as para-cardiac masses [2]. Foremost and commonest are the neoplastic masses. Cystic teratomas are of an embryonic origin and are derived from the three germinal layers in varying degrees. A finding of pericardial anatomical region is common, but in rare cases they have been inspected in myocardial tissue. Pathologically these tumors are multi-cystic [3]. Cardiac myxomas are the most common primary benign cardiac tumor, typically occurring in the left atrium, as solid masses without cystic appearance or cavitations. Case reports of cystic or multi-cystic myxomas the last years are suggesting a new form of myxoma [4]. Furthermore, cardiac lymphangioma is another rare entity in this group which could present as a cystic mass, usually confined to the head and neck, and rarely found in the pericardial region. Intracardiac lymphangioma is exceptionally uncommon. Another intracardiac cavitation structure is thrombus. Intracardiac cystic thrombi are documented in various reports, in all the cardiac chambers. This should be considered in the differential diagnosis as it could masquerade other possible diagnoses [5].
In this case report, the histopathologic examination supported that the cystic structure contained characteristic of chronic inflammation and cardiac abscess as a diagnosis. A cardiac abscess is a localized suppurative infection affecting the myocardium, endocardium, or native valve tissue, and often appears as a cystic lesion. Myocardial abscesses can be classified into three categories based on their clinical associations: those associated with endocarditis, those linked with bacteriaemia, and those with miscellaneous clinical associations [6].
Infective endocarditis (IE) is recognized as the most common cause of cardiac abscess development. The incidence of perivalvular abscess among patients with IE is between 30% and 40%, with the aortic valve having a higher predisposition than the mitral valve and annulus [6, 7]. In this present case, the patient did not exhibit fever, laboratory signs of infection, positive blood cultures, or echocardiographic evidence of vegetation upon admission [7] and IE was clinically rejected [7, 8]. From the cyst’s histopathologic examination, no evidence of endocardial tissue was found, thus excluding the presence of endocardial inflammation (endocarditis) [8].
Bacteremia is the second most prevalent source of a cardiac abscess and can either occur transiently or persistently [6]. Myocardial abscesses that may develop in the site of a prior myocardial infarction site, fermented by the presence of bacteremia, are also encompassed in this group [9]. In contrast to IE, myocardial abscesses resulting from bacteremia are rarely associated with valvular lesions [10, 11]. In our case, blood, urine, and stool cultures yielded negative results. The patient denied any previous or recent signs of infection from his past medical history, and laboratory as well as radiology examinations did not support the presence of an ongoing infection. Furthermore, there are various miscellaneous clinical scenarios that could contribute to the development of myocardial abscesses. Among them, conditions such as trauma, deep burns, and wounds of the thorax, infected pseudoaneurysms, suppurative pericarditis, infected transplanted hearts, extension of infection from sternal abscess, HIV-associated myocarditis and suppuration, parasitic infections and infection of a LV aneurysm or neoplasms are the most common [6, 12, 13]. In this case, the patient did not present macroscopic evidence of thorax’s trauma, burn or infection. Additionally, serological tests excluded HIV-associated myocarditis.
The patient originated from Democratic Republic of Congo where several endemic parasitic diseases are prevalent. The heterogenic group of neglected tropical diseases (NTDs) can affect the heart. Frequently encountered diseases include, schistosomiasis, strongiloidiasis, loiasis, African trypanosomiasis (sleeping sickness), toxoplasmosis, cysticercosis and ecchinococcosis [14-17]. From the above parasitic diseases, only ecchinococcosis, cysticercosis and toxoplasmosis may present with cardiac cystic lesions and potentially lead to cardiomyopathy [18]. The remaining are parasitic diseases that might affect several organs, including cardiac and nervous system tissues, preponderantly unassociated with cystic lesions [18], but with cardiomyopathy. In this case, echinococcosis was excluded based on negative anti-echinococcus IgG antibodies and the absence of typical well-defined hydatic cystic findings in liver, lungs or brain [19, 20]. Toxoplasmosis was also excluded through serological testing, as negative IgG and IgM antibody tests ruled out a possible prior or recent infection [21].
Cysticercosis results from Taenia solium tapeworm egg ingestion. Larvae encystment can occur in almost any tissue and thus lead to different manifestations [22]. Cysticercosis refers to the presence of T. solium cystic larvae throughout the body (e.g., in the muscular system). Taeniasis, by contrast, is caused by adult T. solium tapeworms residing within the intestinal tract. Involvement of T. solium cystic larvae within the central nervous system (CNS) is known as neurocysticercosis (NCC). Taeniasis can be detected through stool microscopy, which was implemented and was not pathological [18, 23]. Epilepsy can be one of the presenting signs of NCC and is considered a leading cause of adult acquired epilepsy worldwide. Seizures result from perilesional inflammation in degenerating cysts and orient with the clinical presentation of this case, where the patient presented with epileptic seizures and newly diagnosed heart failure symptoms.
Cardiac cysticercosis is a silent disease and cardiac involvement is rare. However, autopsy studies have shown a prevalence of 20-25% in patients with concomitant diagnosed NCC. Heart failure as a manifestation is also very rare. Cardiac cysticercosis is known to induce inflammation and myocardial fibrosis. The progression to cardiomyopathy (CMP) in this context is conjectured to be linked with several factors such as the number and location of cysticerci, the duration of infection, the host’s immune respite and the presence of associated complications. Cysticerci are usually multiple and randomly distributed in cardiac tissues, and rarely a single cardiac cyst may be present [24]. It is reasonable to hypothesize that, in the presented case, cysticerci might have exerted an indirect influence on valvular function [25].
The diagnosis of NCC rests on objective evaluation of clinical, radiological, immunological, and epidemiological data collected, based on the diagnostic criteria for human NCC first published in 1996. Brain CT and MRI scans revealed the presence of one cystic lesion in the right frontal lobe which is well defined and has liquid content that has a signal like cerebrospinal fluid (CSF) on CT and MRI. The initial brain CT showed a frontal hypointense cyst without contrast enhancement. The brain MRI T1-weighted images supported a cystic lesion that is isointense to CSF. On T2-weighted images, a cystic lesion isointense to CSF without a surrounding edge is observed. Fluid-attenuated inversion recovery (FLAIRE) images which are also isointense to CSF are seen. All the above are suggesting vesicular stage NCC. No visible scolex was observed in the scan images [25-29].
Collectively, our case fulfils one major neuroimaging criterion: cystic lesions without discernible scolex, and two minor clinical/exposure criteria: clinical manifestations suggestive of NCC and the patient originating from an endemic area of cysticercosis. The precedent described criteria establish NCC as a probable diagnosis [27-29]. The patient was treated with anti-parasitic medication (albendazole) for a period of 6 weeks due to the high suspicion of a parasitic disease. The role of anti-parasitic therapy for controlling seizures associated with NCC remains controversial. The Mondal and Banerjee randomized controlled study, involving 300 patients with NCC, who were observed for several years, came to conclusion of a treatment with anticonvulsants alone, with clinical and radiologic follow-up. The patients in this study who were treated with anthelminthic therapy had an increased occurrence of long-term seizures in the first years, since the complete lesions’ resolution was more likely when cysts are allowed to spontaneously resolve. On the other hand, there are several other randomized controlled trials supporting that anti-helminthic therapy is beneficial for active cyst reduction, particularly during the first weeks [30].
Conclusion
In summary, the present case allows us to consider the parasitic disease spectrum in patients with synchronous myocardial and brain abscesses/cysts. Clinical presentation, microscopic and serological tests, and non-invasive imaging (echocardiography, CT, MRI) are useful tools to support or define the diagnosis. In our case, cysticercosis is considered the more compatible disease with all the clinical evidence collected, though we could not exclude the possibility of a simultaneous occurrence of another disease.
Learning points
Parasitosis should be considered as a possible diagnosis for single or multiple cardiac cystic lesions. Multimodality imaging can be helpful to identify the cause of an endocardial cyst and to detect other synchronous cystic lesions. The treatment of endocardial cystic disease is surgical and should not be delayed to reduce the risk of life-threatening complications.
NCC is one of the most common causes of acquired epilepsy worldwide. New radiological imaging modalities, including advanced MRI methods have improved the radiological diagnosis of NCC.
Acknowledgments
We would like to thank Dr. Anastasios Poyadjis for his advice during the writing of this case report.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Written informed consent was obtained from the patient.
Author contributions
AM and CT participated in patient care, writing of the case report, revisions, and submission process. PA participated in patient care, writing of the case report and revisions. EC and SS participated in writing of the case reports and revisions.
Data Availability
All data in our report were obtained from the patient’s hospitalization. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Beale RA, Russo R, Beale C, Levin W, Atalay MK, Fingleton J, Poppas A, et al. Mitral valve blood cyst diagnosed with the use of multimodality imaging. CASE (Phila). 2021;5(3):173-176.
doi pubmed pmc - Mozaffari Kambiz, Khajali Zahra, Givtaj Nozar, Bakhshandeh Hooman. Intracardiac foregut cyst, in a 42-year-old woman with partial atrioventricular septal defect, a rare incidental finding. Research in Cardiovascular Medicine. 2018 7(3):152-153.
doi - Cohen R, Mirrer B, Loarte P, Navarro V. Intrapericardial mature cystic teratoma in an adult: case presentation. Clin Cardiol. 2013;36(1):6-9.
doi pubmed pmc - Ibanez B, Marcos-Alberca P, Rey M, de Rabago R, Orejas M, Renedo G, Farre J. Multicavitated left atrial myxoma mimicking a hydatid cyst. Eur J Echocardiogr. 2005;6(3):231-233.
doi pubmed - Ouf SG, Abualnaja S, Ghazal SN. A large left ventricular cystic thrombus: unusual presentation of a common entity. CASE (Phila). 2017;1(6):216-220.
doi pubmed pmc - Ramos Tuarez FJ, Yelamanchili VS, Law MA. Cardiac Abscess. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
pubmed - Munoz P, Kestler M, De Alarcon A, Miro JM, Bermejo J, Rodriguez-Abella H, Farinas MC, et al. Current epidemiology and outcome of infective endocarditis: a multicenter, prospective, cohort study. Medicine (Baltimore). 2015;94(43):e1816.
doi pubmed pmc - Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128.
doi pubmed - Xu G, Hu HY. A case of myocardial abscess induced by Staphylococcus aureus with typical electrocardiogram of acute myocardial infarction. Chin Med J (Engl). 2013;126(22):4398.
pubmed - Pasqualucci PL, Aiello VD, Kopel L. Unusual cardiac outcomes of bacteremia due to Staphylococcus aureus infection: a case report. J Med Case Rep. 2012;6:326.
doi pubmed pmc - Garg M, Bhargava J, Garg M, Garg S. Isolated myocardial abscess cavity: An incidental finding on intraoperative transesophageal echocardiography. Ann Card Anaesth. 2021;24(3):411-414.
doi pubmed pmc - Ferreira CR, de Campos FPF. Myocardial abscess as a complication of an infected arteriovenous fistula: autopsy report. Autopsy and Case Reports. 2011;1(3):23-29.
doi - Juliana AE, van 't Kruys KH, Voigt PG, Blom NA. Infectious pseudo-aneurysm of the left ventricle: a case report and a review of the literature. BMC Cardiovasc Disord. 2020;20(1):28.
doi pubmed pmc - Congolese Refugee Health Profile. CDC. www.cdc.gov. March 26, 2021. https://www.cdc.gov/immigrantrefugeehealth/profiles/congolese/index.html#:∼:text=A%20recent%20report%20surveying%2043. Accessed June 08, 2022.
- Gulelat Y, Eguale T, Kebede N, Aleme H, Fevre EM, Cook EAJ. Epidemiology of porcine cysticercosis in Eastern and Southern Africa: systematic review and meta-analysis. Front Public Health. 2022;10:836177.
doi pubmed pmc - Praet N, Kanobana K, Kabwe C, Maketa V, Lukanu P, Lutumba P, Polman K, et al. Taenia solium cysticercosis in the Democratic Republic of Congo: how does pork trade affect the transmission of the parasite? PLoS Negl Trop Dis. 2010;4(9):e817.
doi pubmed pmc - Shonyela SM, Yang G, Wang C. Current status of prevalence, possible control and risk factors associated with porcine cysticercosis from endemic countries in Africa. World Journal of Vaccines. 2018;08(03):53-80.
doi - Hidron A, Vogenthaler N, Santos-Preciado JI, Rodriguez-Morales AJ, Franco-Paredes C, Rassi A, Jr. Cardiac involvement with parasitic infections. Clin Microbiol Rev. 2010;23(2):324-349.
doi pubmed pmc - Sarkari B, Rezaei Z. Immunodiagnosis of human hydatid disease: Where do we stand? World J Methodol. 2015;5(4):185-195.
doi pubmed pmc - Firouzi A, Neshati Pir Borj M, Alizadeh Ghavidel A. Cardiac hydatid cyst: A rare presentation of echinococcal infection. J Cardiovasc Thorac Res. 2019;11(1):75-77.
doi pubmed pmc - Robert-Gangneux F, Guegan H. Anti-Toxoplasma IgG assays: What performances for what purpose? A systematic review. Parasite. 2021;28:39.
doi pubmed pmc - Lyimo FR, Jusabani AM, Makungu H, Mtolera M, Surani S. Thinking outside malaria: a rare case of disseminated cysticercosis with cardiopulmonary involvement from urban tanzania. Cureus. 2021;13(1):e12851.
doi pubmed pmc - Littlewood S. A case report of cardiac cysticercosis in a returning traveller: a rare cause of myocarditis. Eur Heart J Case Rep. 2022;6(5):ytac208.
doi pubmed pmc - Karoline Medina Neri A, Oliveira da Costa Lino D, da Silva Veras S, Pereira Silva R, Bezerra da Silva Junior G. Cardiac cysticercosis: current trends in diagnostic and therapeutic approaches. Current State of the Art in Cysticercosis and Neurocysticercosis. IntechOpen. 2021.
doi - Del Brutto OH, Rajshekhar V, White AC, Jr., Tsang VC, Nash TE, Takayanagui OM, Schantz PM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57(2):177-183.
doi pubmed pmc - Kimura-Hayama ET, Higuera JA, Corona-Cedillo R, Chavez-Macias L, Perochena A, Quiroz-Rojas LY, Rodriguez-Carbajal J, et al. Neurocysticercosis: radiologic-pathologic correlation. Radiographics. 2010;30(6):1705-1719.
doi pubmed - Naveen Kumar. Vidyadhara Rani. MRI evaluation of intracranial cystic lesions. 2017;3(5):151-163.
doi - Garcia HH, O'Neal SE, Noh J, Handali S, Cysticercosis Working Group in P. Laboratory diagnosis of neurocysticercosis (Taenia solium). J Clin Microbiol. 2018;56(9).
doi pubmed pmc - Del Brutto OH, Nash TE, White AC, Jr., Rajshekhar V, Wilkins PP, Singh G, Vasquez CM, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. 2017;372:202-210.
doi pubmed - Das K, Mondal GP, Banerjee M, Mukherjee BB, Singh OP. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. J Clin Neurosci. 2007;14(12):1172-1177.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.








