| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 11, November 2023, pages 356-361
Multiple Complications of Crohn’s Disease and the Need for Early and Continuous Multidisciplinary Undertaking
David Dogahea, d, e , Maxime Taghavia, d, Edouard Cubiliera, Said Sanoussib, Ruth Duttmanc, Joelle Nortiera, Maria do Carmo Filomena Mesquitaa
aNephrology and Dialysis Department, Brugmann University Hospital, Universite Libre de Bruxelles (ULB), Brussels, Belgium
bRadiology Department, Brugmann University Hospital, Universite Libre de Bruxelles (ULB), Brussels, Belgium
cPathology Department, Brugmann University Hospital, Universite Libre de Bruxelles (ULB), Brussels, Belgium
dThese authors contributed equally to this article.
eCorresponding Author: David Dogahe, Nephrology and Dialysis Department, Brugmann University Hospital, Universite Libre de Bruxelles (ULB), 1020 Brussels, Belgium
Manuscript submitted September 11, 2023, accepted October 13, 2023, published online November 23, 2023
Short title: A Case of Crohn’s Disease and UraC
doi: https://doi.org/10.14740/jmc4154
| Abstract | ▴Top |
Crohn’s disease is an inflammatory disease that typically affects the bowels but can also have many different extraintestinal manifestations. One of those complications is immunoglobulin A nephropathy (IgAN), which is one of the most encountered renal lesions in the setting of Crohn’s disease. Another point of focus for Crohn’s patients is the risk of cancer, with a higher risk of colorectal cancer but also extraintestinal neoplasia such as hepatobiliary, hematological, and urinary tract neoplasia. We present the case of a young patient suffering from long-term Crohn’s disease and subsequent IgAN leading to end-stage kidney disease and hemodialysis. The patient was diagnosed young and had undergone multiple surgeries and different treatments in various countries. He then presented in our center already with advanced chronic renal failure from IgAN that was unknown due to poor multidisciplinary follow-up. Shortly after starting hemodialysis, he developed a large abdominal mass, first thought to result from Crohn’s-related fistula. This mass turned out to be a urachal adenocarcinoma, a rare type of bladder cancer with an especially poor prognosis. It is not known whether this type of cancer is associated with either Crohn’s disease or IgAN, and no such association has been previously described. The treatment of urachal cancer usually relies on surgery, with the addition of chemotherapy in some cases. Unfortunately for our patient, his case was already so advanced at the moment of diagnosis that he was excluded from curative treatment and quickly passed away thereafter. This case illustrates many important aspects of the rigorous follow-up that is needed for Crohn’s patients, with regular check-ups, screening investigations, and the need for multidisciplinary evaluation. Furthermore, it describes the development of a rare type of cancer in the setting of Crohn’s disease and IgAN, with no prior established link between these different pathologies.
Keywords: Immunoglobulin A nephropathy; Crohn’s disease; Multidisciplinary evaluation; Urachal cancer
| Introduction | ▴Top |
Crohn’s disease is an inflammatory bowel disease (IBD), with numerous extradigestive manifestations and complications. The prevalence of Crohn’s disease in Western countries is approximately 246.7 cases/100,000 [1].
While digestive symptoms represent the main aspect of the disease, extraintestinal manifestations are frequent and involve many different organs. Urinary lithiasis and associated complications are the most common urinary complications of Crohn’s disease [2-4]. One of the most frequently encountered Crohn’s-related renal lesions in kidney biopsies is immunoglobulin A nephropathy (IgAN), with a prevalence ranging from 16% to 70% depending on ethnicity [5].
Cancer is the second leading cause of mortality in patients with Crohn’s disease, mainly from colorectal cancer (CRC) [1, 6, 7], but Crohn’s patients are also at high risk for some types of extraintestinal neoplasia, most frequently involving the hepatobiliary tract or the hematopoietic system [1, 7].
Urinary tract cancer affects 6.3% of Crohn’s disease patients, the two main types of urinary tract cancers are renal cell carcinoma and bladder cancer [1]. Renal cell carcinoma is more frequent in Crohn’s patients with a severe disease course that starts from a young age. Bladder cancer occurs mainly with long-standing IBD and with the use of some immunosuppressive medications [1].
Urachal cancer is a specific subtype of bladder cancer, derived from the embryological remnant that links the umbilicus to the bladder. It is a rare type of tumor, representing less than 1% of all cases of bladder cancer [8-10]. Urachal cancer has a very poor prognosis and is associated with a 5-year survival rate of less than 50% [10, 11]. This type of cancer has not yet been described in association with Crohn’s disease.
We describe here the case of a patient with Crohn’s disease treated for more than 25 years, on hemodialysis for less than 2 years due to multiple Crohn’s-related nephrological complications, presenting a fatal outcome because of the rapid evolution of urachal cancer.
| Case Report | ▴Top |
Investigations
We present the case of a 43-year-old male of North African origin with a long-standing history of Crohn’s disease. He was diagnosed at age 16 and underwent five consecutive surgeries in Morocco, then in Germany and Belgium for the treatment of his Crohn’s and subsequent complications. The first surgery was a partial colectomy with protective ileostomy and sigmoidostomy before undergoing several subsequent interventions to attempt to reestablish intestinal continuity, culminating in total colectomy and ileostomy.
His medical treatment first consisted of 5-aminosalicylic acid (5-ASA) (mesalazine) regiments for several years before receiving a combination of 5-ASA (2 g, three times daily) with azathioprine (50 mg daily). In October 2018, 5-ASA was suspended because of worsening renal function, and azathioprine was pursued as a monotherapy.
He first consulted our center in January of 2019 with stage 4 chronic kidney disease for evaluation. His blood pressure at the first consultation was 140/80 mm Hg. Blood tests revealed a serum creatinine of 3.29 mg/dL (normal range: 0.7 - 1.2 mg/dL) and a glomerular filtration rate (GFR) estimated at 22 mL/min/1.73 m2 (Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI)). Urine analysis revealed proteinuria of 1.03 g/g creatinine with an albuminuria of 759.9 mg/g creatinine.
A kidney biopsy was performed, with immunofluorescence slides showing strong signals for mesangial IgA and C3 deposits and negative signals for IgM, IgG, C1q, kappa, and lambda deposits. These elements indicated the presence of histological signs suggestive of IgAN. The histological lesions were scored according to the modified Oxford classification as M1-E1-S1-T1-C0 (Fig. 1).
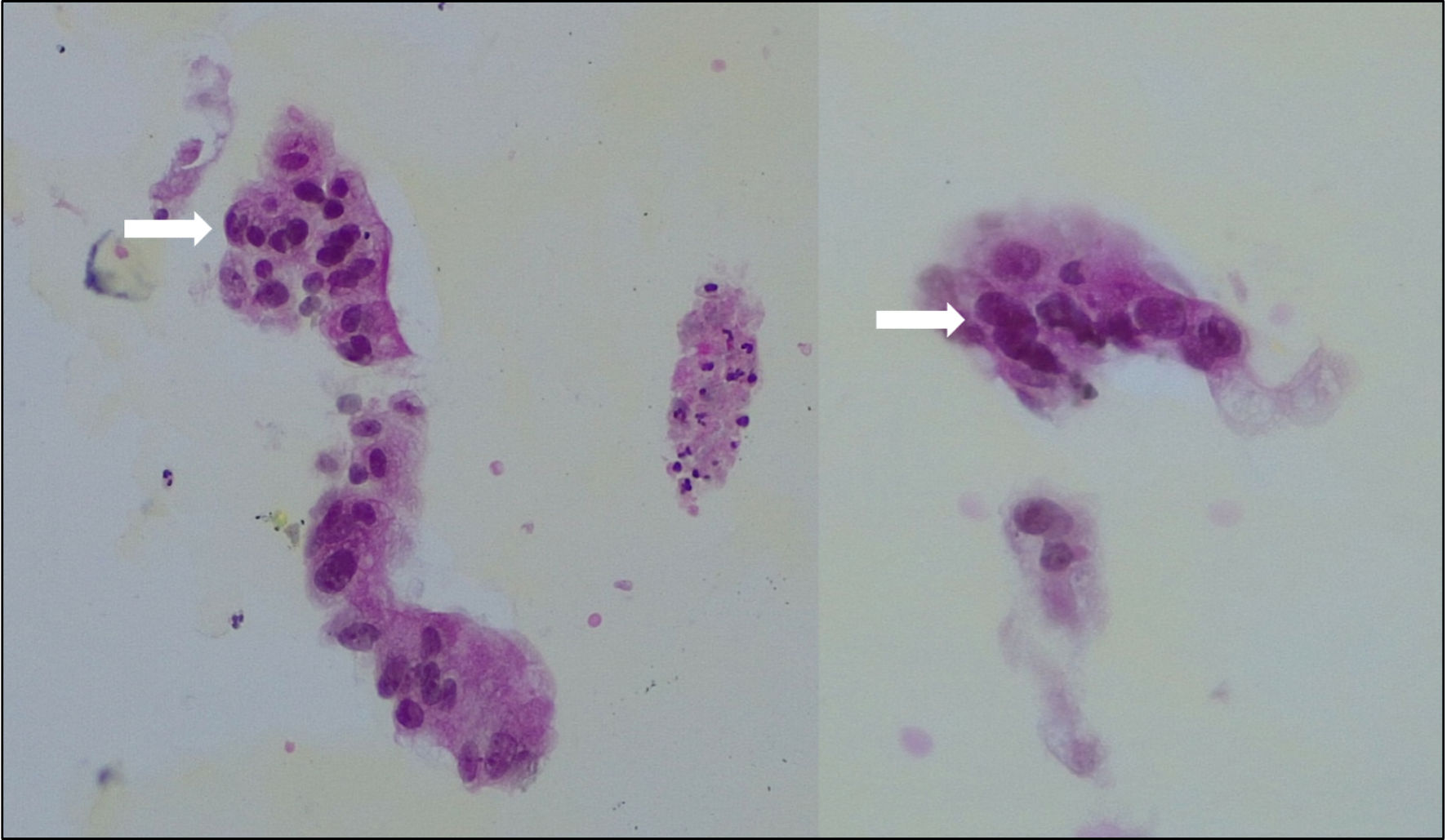 Click for large image | Figure 1. Biopsy of the abdominal mass showing large cylindric cells with important anisonucleosis (arrows), consistent with adenocarcinoma. |
After this initial evaluation, he discontinued his follow-up for several months due to a lack of motivation and the coronavirus disease 2019 (COVID-19) pandemic. He therefore could not benefit from any treatment modification after the results from the biopsy. He did not have any medical follow-up in the meantime and returned in October 2020. By that time, blood tests revealed that he had progressed to end-stage kidney disease (ESKD) with serum creatinine of 6.54 mg/dL and a GFR estimated at 12 mL/min/1.73 m2 (CKD-EPI). Hemodialysis was initiated in January 2021 through a central venous jugular catheter before the creation of an arteriovenous fistula in his left forearm in March 2021. He was estimated to be unfit for peritoneal dialysis because of the several abdominal surgeries he had over the years.
In June 2022, he presented a chronic suprapubic wound with serous discharge, which was first attributed to postoperative scars and thought to be complicated by a fistula. Bacterial swabs revealed an Escherichia coli infection, and topical antiseptic agents were used chronically to clean the wound. The patient, who was self-conscious of the wound, was not present for regular check-up and was also actively hiding the mass from physicians and nurses during hemodialysis sessions. It took several months before proper evaluation of the mass was conducted.
He then presented in December 2022 with the same wound with continuous serous discharge and a large palpable suprapubic mass that was not detectable in previous physical examinations, as well as a weight loss estimated at 10% of total body mass in 3 months and complaints of dysuria.
Diagnosis
Imaging with positron emission tomography and computed tomography (PET-CT) (Fig. 2) showed a voluminous heterogeneous mass along the course of the urachus with heterogeneous fluorodeoxyglucose (FDG) uptake. Pelvic magnetic resonance imaging (MRI) (Fig. 3) performed in December 2022 revealed a large mass following the path of the urachus. It should be noted that this mass was not initially described on a previous abdominal computed tomography (CT) scan in April 2022 (Fig. 4), but retrospective examination of the images demonstrated that the mass was already present.
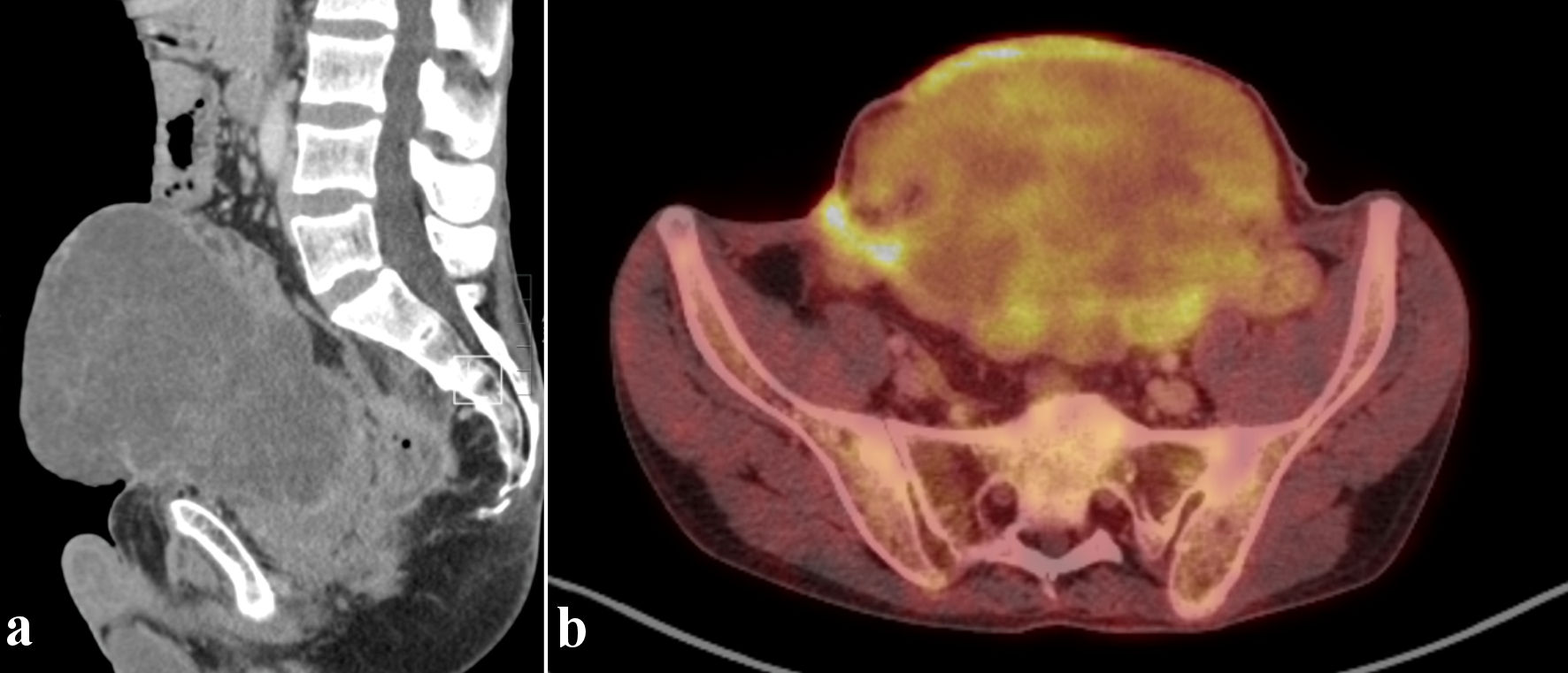 Click for large image | Figure 2. 18F-FDG PET/CT. Sagittal CT view (a) and axial fusion imaging view (b). Voluminous heterogeneous mass along the course of the urachus with heterogeneous FDG uptake. PET/CT: positron emission tomography/computed tomography; FDG: fluorodeoxyglucose; CT: computed tomography. |
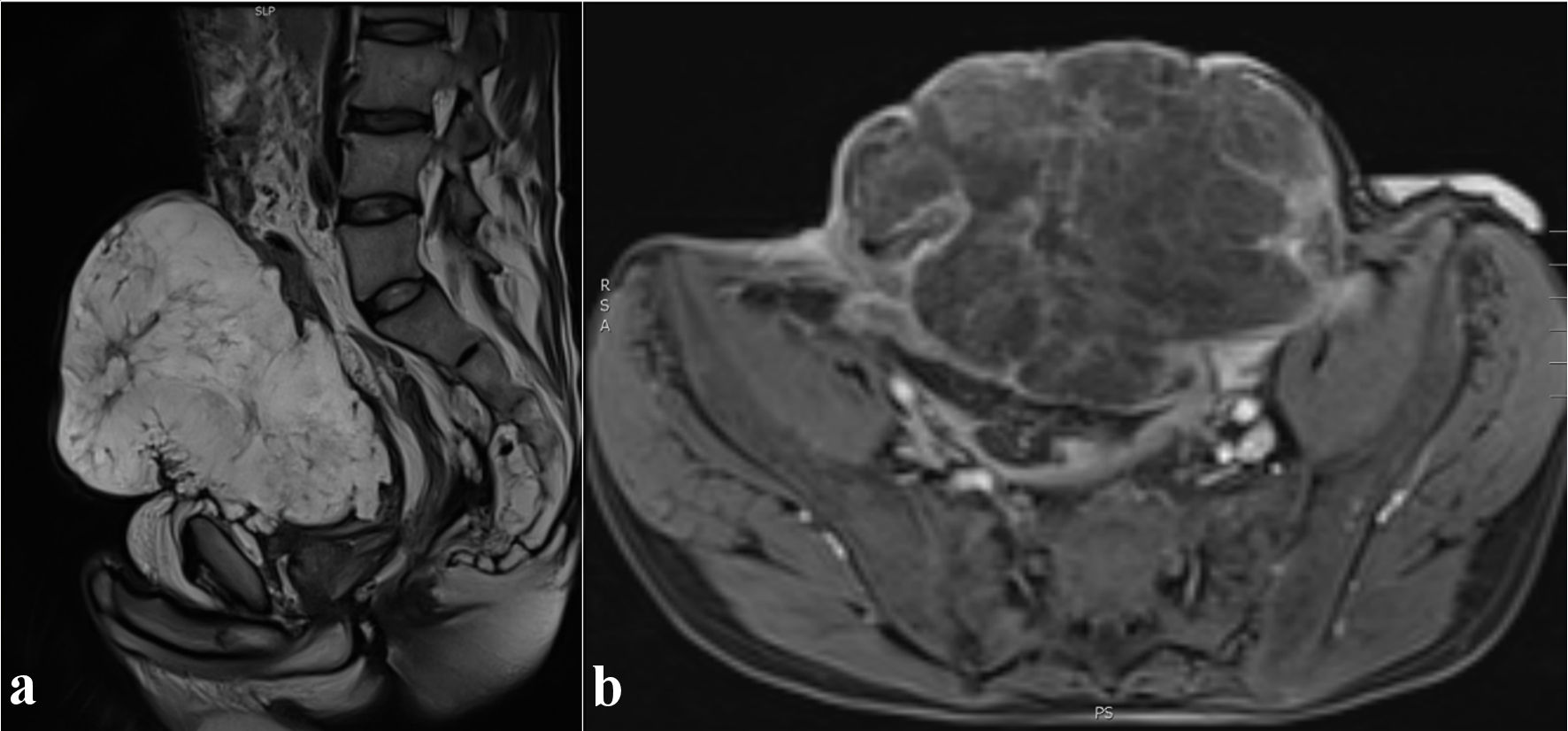 Click for large image | Figure 3. Abdominal MRI. T2-weighted sagittal view (a) and T1-weighted axial view after gadolinium injection (b). Heterogeneous enhancement of the mass. MRI: magnetic resonance imaging. |
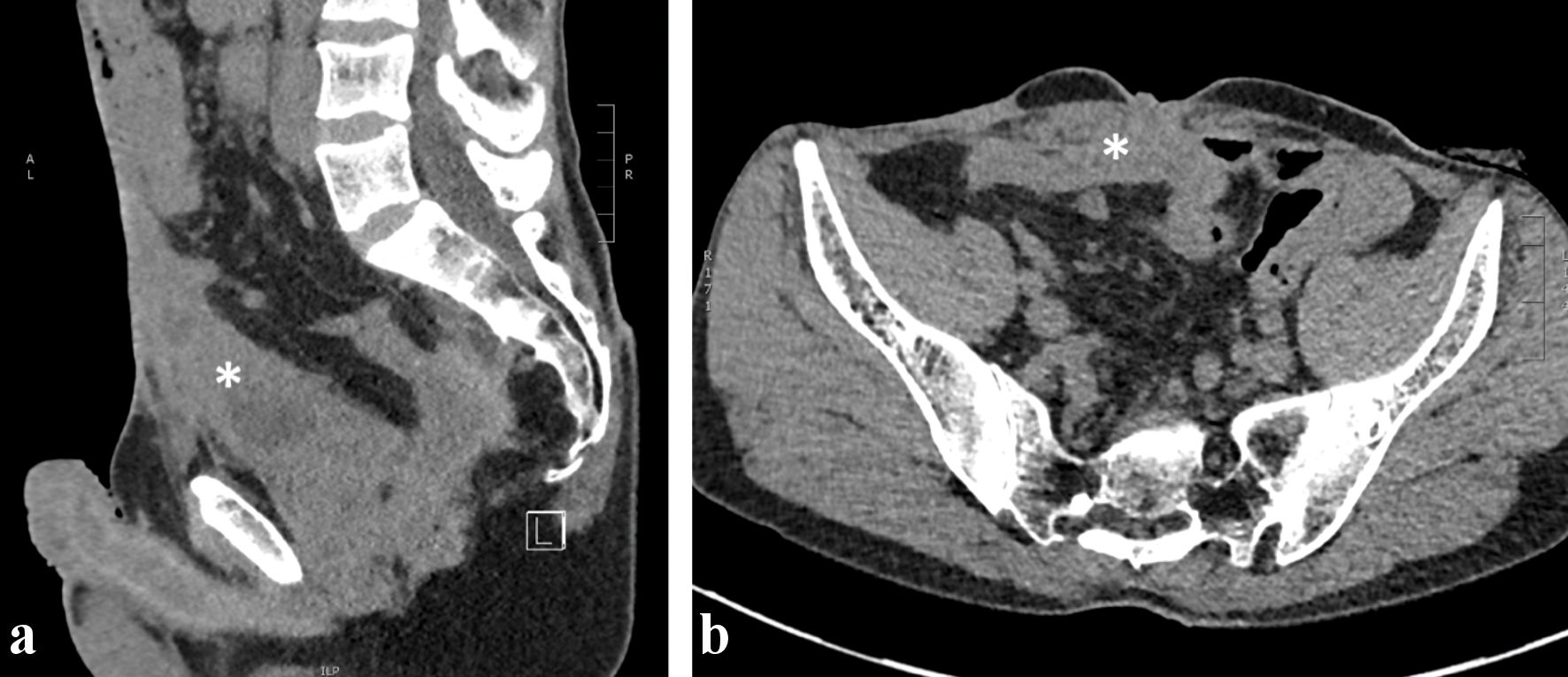 Click for large image | Figure 4. Abdominal CT scan without contrast, sagittal (a) and axial (b) views. Midline heterogeneous solid lesion (asterisk) overhanging the antero-superior wall of the bladder and extending toward the umbilicus. CT: computed tomography. |
This discovery was quickly followed up by an echo-guided transabdominal biopsy showing large cylindric cells with large nuclei and anisonucleosis, consistent with adenocarcinoma.
The case was discussed during a multidisciplinary oncological meeting, with staging IVA, cT4N1Mx.
Treatment and outcomes
Multidisciplinary oncological decision judged the tumor to be too advanced for curative treatment and no specific oncological treatment could be organized. The patient started palliative care and died 2 months later in February 2023.
| Discussion | ▴Top |
This case depicts a patient with long standing history of Crohn’s disease, with multiple complications due to disease progression and poor follow-up, as well as the development of a rare type of cancer in the same patient.
Our patient’s history highlights the necessity for long-term multidisciplinary follow-up of Crohn’s patients. Indeed, many of those patients may develop complications involving the kidneys and urinary tract.
A recent review of renal manifestations in IBD by van Hoeve et al [4] summarized the different types of renal lesions that can be caused by IBD. The most common of these lesions are urinary lithiasis, with oxalate and other types of urinary stones [3, 4]. These patients are also at risk of postrenal obstruction secondary to chronic inflammation and fibrosis of intra-abdominal tissue but also from fistulation and infectious complications [2-4]. Drug-induced nephropathy is another mechanism by which these patients can have renal injury. Almost all medications used to treat IBD can be implicated, although it is most frequently with calcineurin inhibitors. The final causes are the different extraintestinal manifestations of IBD that involve the kidneys. As these are manifestations of the disease itself, they are well correlated with the extent of bowel disease activity [4].
Our patient had a long history of treatment for his Crohn’s disease, with multiple operations and various medical treatments, but there could have been better multidisciplinary follow-up. He arrived in our center already presenting with ESKD and quickly began hemodialysis thereafter. Closer monitoring of extraintestinal complications could have helped detect the IgAN sooner and could have postponed progression to ESKD and the need for dialysis.
IgAN is not rare in the setting of Crohn’s disease [5] and is relatively proportional to the extent of gastrointestinal (GI) inflammation, which promotes IgA1-producing cells, thereby leading to mesangial deposits of IgA [2, 3, 12].
Crohn’s patients with IgAN should benefit from specific treatments to lower proteinuria, slow the progression of disease and improve prognosis [13, 14]. These treatments include angiotensin-converting enzyme (ACE) inhibitors and corticosteroids (such as budesonide). Unfortunately, our patient had very poor nephrological follow-up and such treatment adaptations could not be conducted before it was already too late.
Because of further degradation of renal function, our patient required dialysis. Most patients suffering from ESKD secondary to Crohn’s disease require hemodialysis as IBD patients represent a contraindication to peritoneal dialysis because of GI inflammation, multiple surgeries, and high risk for fistulae and infection [15]. Hence, hemodialysis was proposed to our patient.
Crohn’s disease is associated with higher risk of CRC [1, 6, 7], although some reports found that IBD patients with no prior history of cancer were not associated with a significantly increased risk of colon cancer compared to the general population [16]. They were however at increased risk for certain hematological malignancies [16].
Even though there is mixed evidence on the risk of colon cancer in Crohn’s disease [1, 6, 7, 16, 17], long-term surveillance via ileocolonoscopies is still recommended in the follow-up, as early detection of CRC was found to be an effective way to improve prognosis [17]. Our patient could not benefit from such a follow-up because of a very poor follow-up and several previous surgeries which made it difficult to satisfactorily explore the intestines afterwards. Finally, when he presented for a GI consultation, it was already too late, as he already had a large abdominal mass that turned out to be a urachal cancer.
Urachal cancer is a rare subtype of bladder cancer, with an especially poor prognosis [11]. The histological type is most often adenocarcinoma despite the urachus having a transitional cell epithelium [10, 11]. It often presents with macroscopic hematuria and can also be detected through calcifications of the bladder dome on imaging studies, which is considered to be pathognomonic [9, 10]. The diagnosis of urachal cancer is not standardized but usually relies on the following criteria: 1) Tumor located in the dome or anterior bladder wall; 2) Tumor growth in the bladder wall; 3) Absence of atypical intestinal metaplasia and cystitis beyond the dome/anterior wall; 4) Absence of urothelial bladder neoplasia; 5) Exclusion of a primary adenocarcinoma of a different origin [8]. New techniques have also been developed such as urinary fluorescence in situ hybridization (FISH) (UroVysion) to differentiate benign from malignant urachal tissue [8, 9].
Our patient satisfied these diagnostic criteria. He presented with such a large abdominal mass that a pathological sample could be obtained by direct transcutaneous puncture. Usually, urachal cancer samples are obtained through cystoscopy [9].
Although there is no standardized protocol for urachal cancer, its treatment usually involves surgery and additional chemotherapy in some cases. Regiments 5-fluorouracil (5-FU) and FOLFOX (5-FU, oxaliplatin, and leucovorin) have both been described as effective in urachal cancer cases, which makes sense considering the similar profile of urachal cancer to CRC [10, 18]. Indeed, next-generation sequencing studies have revealed that urachal cancer has a genomic profile that closely resembles that of CRC [18]. This could be interesting because as previously discussed, CRC seems to be one of the cancers that is at increased risk in Crohn’s disease patients. This potential link could have both meaning for risk assessment and follow-up of Crohn’s disease patients but also as therapeutic options for urachal cancer [18].
Our patient’s baseline oncological setting at diagnosis was already so advanced with this very poor prognosis tumor that curative treatment was directly excluded by multidisciplinary oncological discussion. Investigations were stopped and palliative comfort-oriented care was therefore organized. The patient quickly deteriorated and passed away 2 months thereafter.
An underlying association between Crohn’s disease and urachal cancer could be at stake but would require a larger case series for confirmation, and its clinical utility remains unclear considering its poor prognosis despite adequate treatment.
There are a few reports of Crohn’s disease with urachal fistula and infection, but there is, to the best of our knowledge, no report of Crohn’s or IBD patients presenting with urachal cancer. We know that IBD is a risk factor for certain types of tumors, and bladder cancer seems to have a clearly increased prevalence in this population [1], although none have been reported with urachal tumors.
Learning points
Crohn’s disease is a common disease with chronic repercussions and frequent renal involvement. IgAN is one of the most frequent types of nephropathies encountered in the setting of IBD. Active surveillance of nephrological involvement is recommended as part of the multidisciplinary approach.
This disease can evolve over a lifetime and have both physical and psychological impacts on the patient’s life. It is essential to closely monitor these patients and offer them support for all these different aspects. The risk of cancer is increased with IBD, mainly colorectal, but also hepatobiliary and hematopoietic cancer. Regular screening of Crohn’s patients is therefore a must for long-term follow-up. Urachal cancer is rare but aggressive and has a poor prognosis. Early detection is therefore essential for its treatment. A further case series would be needed to see if a link between Crohn’s disease and urachal cancer could exist.
Acknowledgments
None to declare.
Financial Disclosure
This study was conducted at Brugmann University Hospital and received no specific funding.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained directly from the patient.
Author Contributions
DD, MT, EC, and MM participated in the collection of clinical data and in writing the manuscript. SS provided the radiological images and their description. RD provided the pathology slides and their description. JN and MM supervised the writing and reviewing of the manuscript. All authors have read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
IgAN: immunoglobulin A nephropathy; CD: Crohn’s disease; IBD: inflammatory bowel disease; CRC: colorectal cancer; GFR: glomerular filtration rate; ESKD: end-stage kidney disease; ACE: angiotensin-converting enzyme; GI: gastrointestinal; UraC: urachal cancer
| References | ▴Top |
- Mala A, Foteinogiannopoulou K, Koutroubakis IE. Solid extraintestinal malignancies in patients with inflammatory bowel disease. World J Gastrointest Oncol. 2021;13(12):1956-1980.
doi pubmed pmc - Filiopoulos V, Trompouki S, Hadjiyannakos D, Paraskevakou H, Kamperoglou D, Vlassopoulos D. IgA nephropathy in association with Crohn's disease: a case report and brief review of the literature. Ren Fail. 2010;32(4):523-527.
doi pubmed - Terasaka T, Uchida HA, Umebayashi R, Tsukamoto K, Tanaka K, Kitagawa M, Sugiyama H, et al. The possible involvement of intestine-derived IgA1: a case of IgA nephropathy associated with Crohn's disease. BMC Nephrol. 2016;17(1):122.
doi pubmed pmc - van Hoeve K, Hoffman I. Renal manifestations in inflammatory bowel disease: a systematic review. J Gastroenterol. 2022;57(9):619-629.
doi pubmed - Yang Z, Xu X, Dong Y, Zhang Y. The pathological and outcome characteristics of renal lesions in Crohn's disease. BMC Nephrol. 2022;23(1):256.
doi pubmed pmc - Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19(4):789-799.
doi pubmed - Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):378-389.
doi pubmed pmc - Loizzo D, Pandolfo SD, Crocerossa F, Guruli G, Ferro M, Paul AK, Imbimbo C, et al. Current management of urachal carcinoma: an evidence-based guide for clinical practice. Eur Urol Open Sci. 2022;39:1-6.
doi pubmed pmc - Li S, Meng X, Liang P, Feng C, Shen Y, Hu D, Li Z. Clinical and radiological features of urachal carcinoma and infection. Front Oncol. 2021;11:702116.
doi pubmed pmc - Szarvas T, Modos O, Niedworok C, Reis H, Szendroi A, Szasz MA, Nyirady P. Clinical, prognostic, and therapeutic aspects of urachal carcinoma-A comprehensive review with meta-analysis of 1,010 cases. Urol Oncol. 2016;34(9):388-398.
doi pubmed - Sellars H, Macleod C, Perakath B. Ileal perforation and fistulated urachal remnant in Crohn's disease. N Z Med J. 2019;132(1507):100-103.
pubmed - Angeletti A, Arrigo S, Madeo A, Molteni M, Vietti E, Arcuri L, Coccia MC, et al. Different renal manifestations associated with very early onset pediatric inflammatory bowel disease: case report and review of literature. BMC Nephrol. 2021;22(1):146.
doi pubmed pmc - Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1-S276.
doi pubmed - Doumas SA, Tsironis C, Bolaji AA, Garantziotis P, Frangou E. Glomerulonephritis and inflammatory bowel disease: A tale of gut-kidney axis dysfunction. Autoimmun Rev. 2023;22(6):103327.
doi pubmed - Li PK, Chow KM. Peritoneal dialysis patient selection: characteristics for success. Adv Chronic Kidney Dis. 2009;16(3):160-168.
doi pubmed - Wang JH, D'Arcy M, Barnes EL, Freedman ND, Engels EA, Song M. Associations of inflammatory bowel disease and subsequent cancers in a population-based study of older adults in the United States. JNCI Cancer Spectr. 2022;6(1):pkab096.
doi pubmed pmc - Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1-s106.
doi pubmed pmc - Benjamin DJ, Kalebasty AR. Treatment approaches for urachal cancer: Use of immunotherapy and targeted therapies. Rare Tumors. 2023;15:20363613231189984.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.








