| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 5, May 2013, pages 296-299
A Case of Severe Hypernatremic Myopathy by Primary Hypodipsia, Hyperglycemic Hypertonic State in a 17-Year-Old Patient With Mental Retardation
Chan Sung Parka, Won Beom Kimb, Hyun Soeng Leea, Young IL Kima, IL Sung Nam-Goonga, Eun Sook Kima, c
aDepartment of Internal Medicine, Ulsan University Hospital, College of Medicine University of Ulsan, Ulsan, Korea
bFamily Medicine, Ulsan University Hospital, College of Medicine University of Ulsan, Ulsan, Korea
cCorresponding author: Eun Sook Kim, Department of Internal Medicine, Ulsan University Hospital, 290-3 Junha-Dong, Dong-gu, Ulsan, Korea
Manuscript accepted for publication February 21, 2013
Short title: Severe Hypernatremic Myopathy with HHS
doi: https://doi.org/10.4021/jmc1136w
| Abstract | ▴Top |
Although severe hypernatremia, defined as a sodium concentration > 180 mEq/L, is associated with a high mortality rate, particularly in adults, little is known about severe hypernatremia in patients with diabetic ketoacidosis (DKA). Hypernatremic myopathy has been reported in patients with severe dehydration, acute kidney injury, and rhabdomyolysis. Dehydration in a diabetic patient with mental retardation may be caused by osmotic diuresis and exacerbated by hypodipsia, which requires the patient to be educated about regular oral hydration. Here, we describe a 17-year-old patient with reversible severe hypernatremia (serum corrected sodium, 180 mEq/L) and primary hypodipsia, but without DKA. The patient visited the emergency room due to agitation and weakness in both lower extremities. Initial laboratory tests showed hyperglycemia, acute kidney injury, acute hepatitis, and myopathy. Following rehydration with one-quarter isotonic saline and intensive insulin therapy, the patient’s symptoms (including leg weaknesses) and laboratory findings improved.
Keywords: Severe hypernatremia; Myopathy; Hypodipsia; Hyperglycemia
| Introduction | ▴Top |
Severe hypernatremia, defined as a sodium concentration > 180 mEq/L, is associated with a high mortality rate, particularly in adults. Moreover, children with hypernatremia are estimated to carry a 15-fold higher risk of death than those with no hypernatremia [1]. Severe and extreme hypernatremia are defined as serum sodium concentrations > 160 mmol/L and > 190 mmol/L, respectively [2]. There are few studies reporting severe hypernatremia in patients with diabetic ketoacidosis (DKA) or myopathy associated with hypernatremia. Hypernatremic myopathy is associated with severe dehydration, acute kidney injury, and rhabdomyolysis [2]. In diabetic patients with mental retardation, dehydration can be caused by osmotic diuresis and is exacerbated by hypodipsia. Here, we describe a 17-year-old patient with reversible severe hypernatremia (serum corrected sodium, 180 mEq/L) with primary hypodipsia and myopathy, but without DKA.
| Case Report | ▴Top |
A 17-year-old patient presented to the emergency room of our hospital with a 3 day history of agitation and weakness in both legs. He had been admitted twice before due to similar recurrent symptoms. During his most recent admission, he was diagnosed with mental retardation, dysgenesis of the corpus callosum (Fig. 1), and complete cleft palate; however, he had a normal 46XY karyotype. Two years earlier, he was diagnosed with type 2 diabetes mellitus, but his parents stopped his oral antidiabetic drugs because they caused significant discomfort. Upon physical examination, he was alert but uncooperative due his mental problems. He showed grade III motor weakness with a positive Gowers’ sign, as well as decreased deep tendon reflexes at both knees. He did not complain of thirst.
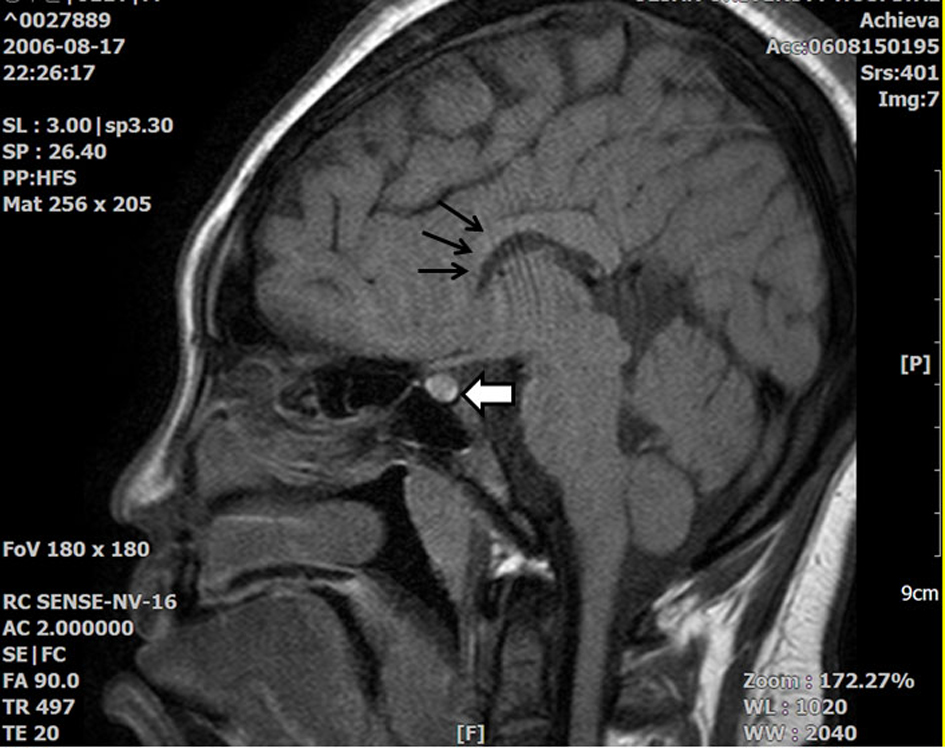 Click for large image | Figure 1. T1 Sella MRI showing that the high signal intensity in the posterior pituitary gland decreased slightly, but did not disappear (white thick arrow). There is suspicious partial agenesis or atrophy of the rostral portion of the corpus callosum (black thin arrow). |
Initial laboratory findings showed hyperglycemia (serum glucose, 695 mg/dL), acute kidney injury (serum creatinine, 1.86 mg/dL; estimated GFR, 50.54 mL/min), acute hepatitis (serum AST, 61 IU/L; ALT, 117 IU/L), myopathy (serum CK, 762 IU/L), high serum (420 mOs/kg) and urinary (899 mOs/kg) osmolarity. Initial arterial blood gas analysis showed the following: pH, 7.4; PCO2, 43 mmHg; HCO3, 28.4 mM/L; and oxygen saturation, 94%. Laboratory findings regarding diabetes were as follows: fasting plasma glucose, 577 mg/dL; post-prandial plasma glucose, 726 mg/dL; hemoglobin A1c, 7.5%; and plasma C-peptide, 2.40 ng/mL. There was no evidence of DKA.
We started hydration with half-isotonic saline and treated the diabetes with multiple daily insulin injections. The patient’s serum sodium concentration increased after 1 day. Therefore, we changed the hydration fluid to one-quarter isotonic saline. After several days of hydration and intense insulin therapy, his symptoms (including the leg weakness) resolved, and his serum sodium and CK concentrations and osmolarity returned to normal (Table 1) (Fig. 2).
 Click to view | Table 1. Laboratory Findings During Hospitalization |
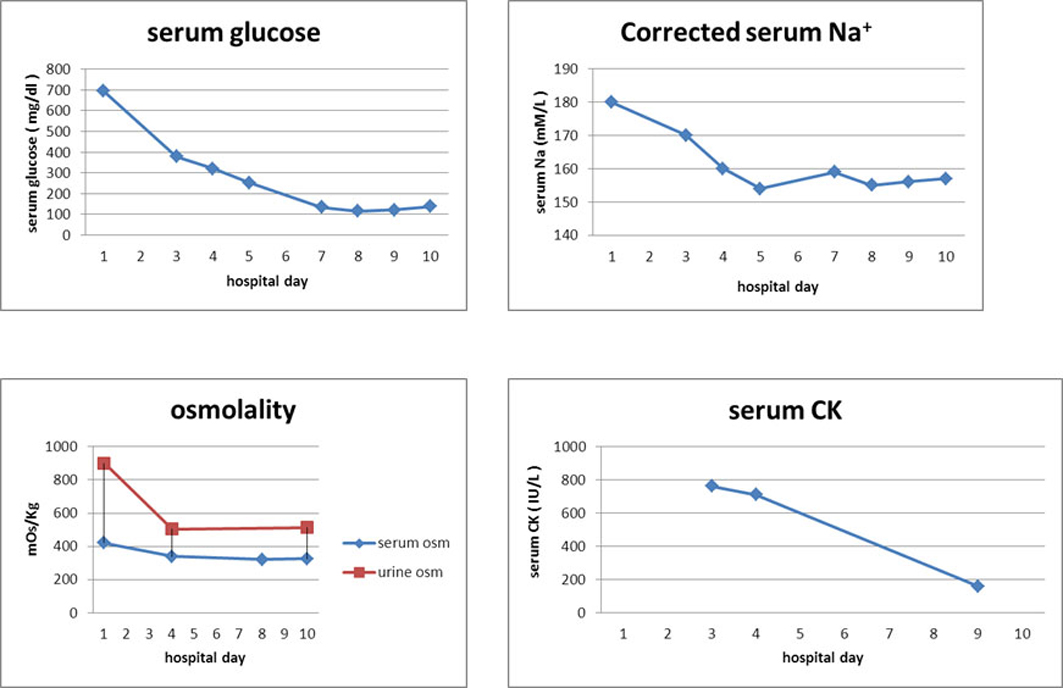 Click for large image | Figure 2. After hydration (from HD1 to HD10), the concentrations of Na and CK in the patient’s serum and osmolarity improved. HD, hospital day; Na, sodium; osm, osmolarity; CK, creatine phosphokinase. |
| Discussion | ▴Top |
Most patients with DKA have low serum sodium concentrations, although correction for hyperglycemia usually brings the sodium concentration back within the normal range. Serum sodium may be abnormally high when a patient is severely dehydrated [3]. Obese pediatric patients with type II diabetes, who initially present with hyperosmolar hyperglycemia, either with or without ketoacidosis, may also present with mild to moderate hypernatremia, often caused by severe dehydration [4]. Since the current patient showed mental retardation, with an impaired thirst center and hyperosmolar hyperglycemia, he did not drink enough; therefore, he presented with severe hypernatremia.
Although some case studies describe patients with hypernatremic myopathy, this condition is not fully understood. Hypernatremic myopathy is characterized by a young age-of-onset. Patients are predominantly male, with remittent or episodic symmetric proximal muscle weakness (Gowers’ sign) and myalgia (either with or without stiffness) that lasts for hours or days. The respiratory, facial and esophageal muscles are spared and there is no muscle atrophy. Deep tendon reflexes and sensory function are normal, but the patients show lethargy and/or general malaise and have dry skin [5]. Muscle weakness in patients with hypernatremic myopathy may be caused by the depletion of intramuscular energy stores, probably due to an overactive Na-K pump, which is attempting to correct the intramuscular electrolyte imbalance [6]. However, the plasma sodium concentration that induces myopathy has not been determined, although patients with myopathy associated with hypernatremia have plasma sodium concentrations of 160 - 180 nmol/L [7]. These patients also have plasma CK concentrations ranging from 500 - 60,000 IU/L, with many also showing acute renal insufficiency [7].
Hypernatremic myopathy may be diagnosed from typical myopathic symptoms and sustained hypernatremia, which show prompt symptomatic improvement when the hypernatremia is controlled [5]. Other compatible clinical and laboratory findings may help the diagnosis (but are not necessary), and head CT/MRI may be performed to identify any hypothalamic lesions [5].
Adipsia can aggravate the dehydration and influence the occurrence of hypernatremia. Adipsic hypernatremia is associated with several intracranial pathologies, mostly in or around the hypothalamus [8]. One study summarized the findings in four patients with adipsic hypernatremia associated with the abnormal development of the corpus callosum [6]. Chromosomal abnormalities are associated with dysgenesis of the corpus callosum and cleft palate, but no chromosomal abnormality was detected in our patient [9-11].
Treatment for hypernatremic myopathy involves rehydration and treatment of the underlying disease [5]. Following hydration with one-quarter isotonic saline and intense insulin therapy, the current patient’s symptoms resolved completely and the laboratory findings were normal.
In summary, dehydration in a diabetic patient with mental retardation may be caused by osmotic diuresis and be exacerbated by hypodipsia, resulting in severe hypernatremia. It is important that these patients receive intensive education about regular oral hydratio.
Conflicts of Interest
There were no conflicts of interest.
| References | ▴Top |
- Moritz ML, Ayus JC. Disorders of water metabolism in children: hyponatremia and hypernatremia. Pediatr Rev. 2002;23(11):371-380.
pubmed - Yang TY, Chang JW, Tseng MH, Wang HH, Niu DM, Yang LY. Extreme hypernatremia combined with rhabdomyolysis and acute renal failure. J Chin Med Assoc. 2009;72(10):555-558.
doi - Wolfsdorf J, Glaser N, Sperling MA. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
doi pubmed - Bhowmick SK, Levens KL, Rettig KR. Hyperosmolar hyperglycemic crisis: an acute life-threatening event in children and adolescents with type 2 diabetes mellitus. Endocr Pract. 2005;11(1):23-29.
pubmed - Huang MN, Chen JJ, Lee KL, Tseng FY, Yu CL, Hsieh SC. Hypernatremic myopathy caused by a hypothalamic mixed germ cell tumor mimicking polymyositis. Clin Rheumatol. 2007;26(9):1591-1594.
doi pubmed - Komatsu H, Miyake H, Kakita S, Ikuta H. Hypoplasia of the corpus callosum associated with adipsic hypernatremia and hypothalamic hypogonadotropinism: a case report and review of the literature. Pediatr Int. 2001;43(6):683-687.
doi pubmed - Zantut-Wittmann DE, Garmes HM, Panzan AD, Lima Mde O, Baptista MT. Severe rhabdomyolysis due to adipsic hypernatremia after craniopharyngioma surgery. Arq Bras Endocrinol Metabol. 2007;51(7):1175-1179.
doi pubmed - Baylis PH, Thompson CJ. Osmoregulation of vasopressin secretion and thirst in health and disease. Clin Endocrinol (Oxf). 1988;29(5):549-576.
doi - Savasta S, Chiapedi S, Perrini S, Tognato E, Corsano L, Chiara A. Pai syndrome: a further report of a case with bifid nose, lipoma, and agenesis of the corpus callosum. Childs Nerv Syst. 2008;24(6):773-776.
doi pubmed - Christensen RD, Yaish HM. A neonate with the Pelger-Huet anomaly, cleft lip and palate, and agenesis of the corpus callosum, with a chromosomal microdeletion involving 1q41 to 1q42.12. J Perinatol. 2012;32(3):238-240.
doi pubmed - Winters J, Markello T, Nance W, Jackson-Cook C. Mosaic "tetrasomy" 8p: case report and review of the literature. Clin Genet. 1995;48(4):195-198.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


