| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 7, July 2013, pages 466-470
Rapid Remission of a Second Primary Kaposi’s Sarcoma With Thalidomide Treatment in a Patient Receiving Adjuvant Chemotherapy for Colon Cancer
Chih-Ming Choua, e, Kuei-Fang Choub, e, Wei-Yu Chenc, En-Kwang Lind, Yi-Fang Changb, Ying-Wen Sub, f
aDivision of Hematology and Medical Oncology, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, No.111, Section 3, Hsing-Long Rd, Taipei 116, Taiwan
bDivision of Hematology and Medical Oncology, Department of Internal Medicine, Mackay Memorial Hospital, No.92, Section 2, Zhongshan North Road, Taipei 104, Taiwan
cDepartment of Pathology, and Wan Fang Hospital, Taipei Medical University, No.111, Section 3, Hsing-Long Rd, Taipei 116, Taiwan
dDivision of Colorectal Surgery, Department of Surgery , Wan Fang Hospital, Taipei Medical University, No.111, Section 3, Hsing-Long Rd, Taipei 116, Taiwan
eThese authors contributed equally to this work
fCorresponding author: Ying-Wen Su, Division of Hematology and Medical Oncology, Department of Internal Medicine, Mackay Memorial Hospital, No.92, Section 2, Zhongshan North Road, Taipei 104, Taiwan
Manuscript accepted for publication March 21, 2013
Short title: Kaposi’s Sarcoma During Chemotherapy for Colon Cancer
doi: https://doi.org/10.4021/jmc1199w
| Abstract | ▴Top |
Kaposi’s sarcoma is a human herpes virus 8 associated-angioproliferative disorder, which is commonly associated with immunocompromised conditions such as those which arise in renal transplant recipients, or in acquired immune-deficiency syndrome (AIDS) cases. It is less well described, however, in patients with solid tumors undergoing adjuvant chemotherapy after surgery. We report the case of an HIV-negative heterosexual Taiwanese man who developed a second primary Kaposi’s sarcoma 5 months after the start of adjuvant chemotherapy for stage III colon cancer. Thalidomide treatment was started after the patient completed 6 months of adjuvant chemotherapy, and the Kaposi’s sarcoma regressed within 2 months. The case reported here suggests that pre-existing HHV-8 infection and adjuvant chemotherapy may predispose these patients to a second malignancy with Kaposi’s sarcoma. Restoration of immune function through cessation of chemotherapy and thalidomide administration may accelerate the regression of the disease, thereby avoiding the need for radiotherapy or anthracycline-based chemotherapy.
Keywords: Kaposi’s sarcoma; Colon cancer; Thalidomide
| Introduction | ▴Top |
Kaposi’s sarcoma (KS) is an angioproliferative disorder that occurs rarely in Asian countries. It is clinically classified into 4 types: (I) classic, mainly occurring in elderly men of Mediterranean or East European origin; (II) African-endemic; (III) iatrogenic, developing in solid organ transplantation recipients; and (IV) acquired-immune-deficiency-syndrome (AIDS)–associated [1]. In addition to the strong association with human herpes virus 8 (HHV-8), immunosuppression, such as that resulting from immunosuppressant use in allograft recipients, or arising from human immunodeficiency virus (HIV) infection, is a well described risk factor for the development of KS. In a retrospective, population-based study that investigated classic KS as a second primary malignancy in an HIV-negative population, development of KS was significantly greater in patients with lymphohematopoietic malignancies such as lymphoma and leukemia with odds ratios (OR) of 7.5 and 5.3, respectively [2]. Among other solid tumor patients, only breast cancer patients were found to have an increased risk of development of KS (OR = 2.2). Cancer patients with a first primary in the lung, colon, stomach, larynx, liver, pancreas, or kidney showed secondary KS less frequently.
Here, we report the case of a Taiwanese patient who developed HIV-negative classic KS after receiving 5 months of adjuvant chemotherapy for stage III colon cancer. After cessation of chemotherapy, the cutaneous lesions regressed dramatically within 2 weeks of starting thalidomide treatment.
| Case Report | ▴Top |
This 65 year-old man was diagnosed with sigmoid colon cancer, characterized as well-to moderately differentiated adenocarcinoma, during regular health checkups in July 2011. No history of homosexuality, multiple sexual partners, or drug abuse was identified. An HIV (Human immunodeficiency virus) screening test was negative. He had no past medical history except for a thalassemia trait. After surgery for colon cancer, the tumor was pathologically staged as pT2N1M0, stage IIIA, and adjuvant chemotherapy with 5-fluorouracil and oxaliplatin was started 3 weeks after the operation. The chemotherapy regimen followed the modified FOLFOX6 protocol [3] and dexamethasone 10 mg was included prior to each cycle of chemotherapy as a part of the prophylactic antiemetic. The 6 months of adjuvant chemotherapy was tolerated very well with only mild chemotherapy-induced nausea and vomiting. During the third month of treatment, grade 1 anemia, increased circulating monocytes (up to 9%), myelocytes (1%) and normoblasts (1 per 100 counted WBCs) were noted briefly for 2 months. A bone marrow exam showed only slightly hypercellular bone marrow with a cellularity of 35%, and there was no abnormal cell infiltration in the marrow. After 4.5 months of chemotherapy, two violaceous indurated plaques up to 2 × 2 cm were noted on the medial dorsal surface of his left foot (Fig. 1a). Two weeks later, a skin biopsy was taken and pathology revealed these to be Kaposi’s sarcoma. The tumor cells from the sarcoma were immunochemically positive for human herpes virus 8 (Fig. 2). Repeated endoscopy to evaluate for visceral mucosal involvement was negative. Other systemic workups showed no evidence of metastasis. Since the lesions were restricted to his left lower leg initially, he was referred for local radiation while the adjuvant chemotherapy was maintained. While waiting for radiotherapy (5.5 months after adjuvant chemotherapy started), his left lower leg became increasingly edematous and a new skin lesion, similar to that previously observed, was found on his right lower leg. Immediately after the 6-month course of adjuvant chemotherapy was complete, thalidomide 100 mg per day was given orally. The main lesions became flattened two weeks after thalidomide treatment and completely disappeared after 2 months of treatment (Fig. 1 b-d). Thalidomide was used for a total of 3 months and the patient is still in complete remission after 6 months of observation.
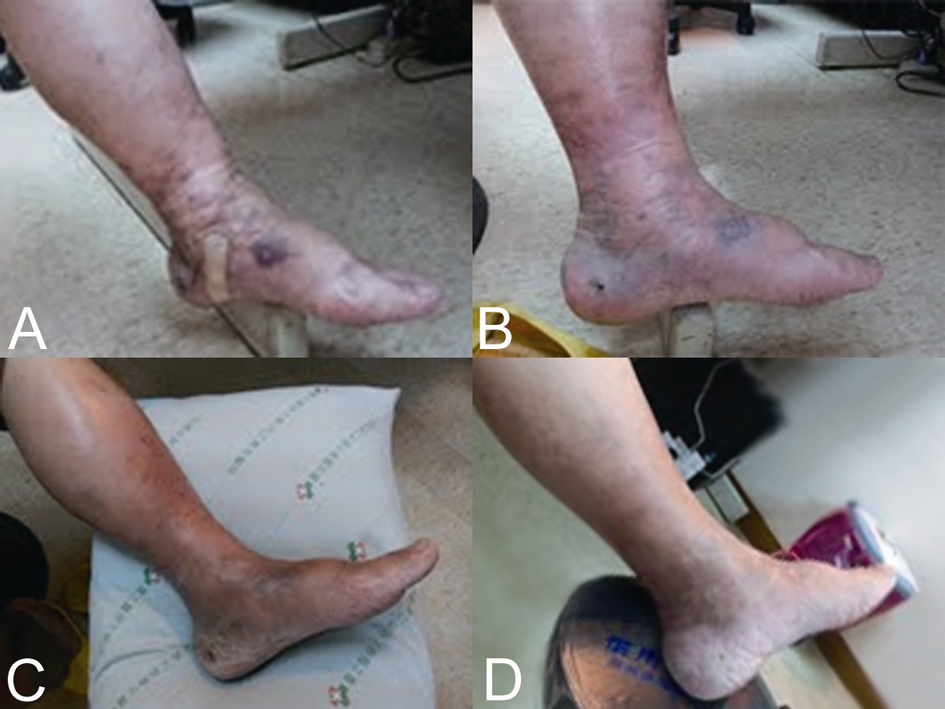 Click for large image | Figure 1. Kaposi’s sarcoma on the left foot before (a) and after thalidomide treatment for 2 weeks (b), 4 weeks (c) and 8 weeks (d). |
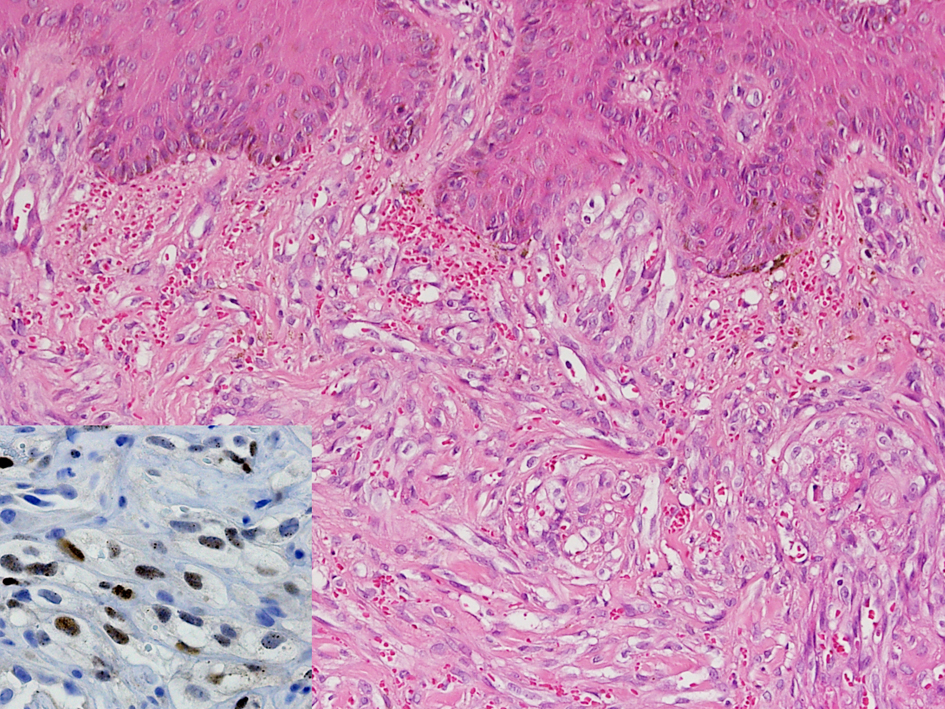 Click for large image | Figure 2. Histological analysis of the Kaposi’s sarcoma biopsy. The tumor is composed of bland-appearing spindle tumor cells extensively involving the dermis. Slit-like spaces, containing erythrocytes, exist between the tumor cells (Hematoxyline & eosin, original magnification ×200). Immunoreactivity with human herpes virus-8 in the tumor cells confirms the diagnosis (Inset, original magnification ×400). |
| Discussion | ▴Top |
Here we report a secondary cutaneous Kaposi’s sarcoma arising in an HIV-negative Asian patient during adjuvant chemotherapy for colon cancer. The disease progressed aggressively during the 5th and 6th months of adjuvant chemotherapy and regressed rapidly after thalidomide treatment and cessation of chemotherapy.
Before the era of AIDS, it was well known that the incidence of KS development increased following hematopoietic malignancies [4]. However, the development of KS after solid tumors, other than lymphoma, does not occur as frequently. As discussed earlier, only patients with breast cancer showed a modestly increased risk of developing a secondary malignancy of Kaposi’s sarcoma in a single population-based retrospective study [2]. For colorectal cancer patients, the risk in the studied population was not significantly increased in comparison with control cases (OR = 0.5, 0.2 - 1.0).
One possible explanation for the heterogeneous association of secondary KS and different solid tumors may lie in the radiotherapy and chemotherapy treatment of the primary neoplasm. Given that the potency of immunosuppression is a highly relevant factor for the development of KS in patients receiving renal transplant [5], patients receiving more intense or longer chemotherapy protocols may similarly be at a significantly higher risk for secondary KS. Although it is well established that KS is commonly associated with an immunocompromised condition, it is less well described in patients undergoing adjuvant chemotherapy.
Another independent risk factor for the development of KS is a pre-existing infection with HHV-8, also known as Kaposi’s sarcoma-associated herpes virus (KSHV). HHV-8 genes have been found in every type of KS [6]. In patients with a renal transplant, seropositive recipients had a 28 - 75 fold higher rate of KS occurrence than did seronegative recipients, suggesting that reactivation of HHV-8 may play a role in KS development [7]. Global HHV-8 infection rates vary widely, but they are very high in endemic areas, including sub-Saharan Africa (where the seropositive rate is 50-70%) and the Mediterranean region (20-30%) [8]. Although the prevalence of HHV-8 in Asians was not reported as being as high as that of endemic areas, it is not uncommon [9]. In Taiwanese blood donors, the seroprevalence was between 7.8% and 19.6 % depending on the detection method used [10, 11]. In some ethnic groups in China, an increased prevalence with respect to the general population has been reported [12]. The life cycle of HHV-8 has two distinct phases: inactive latency and active, lytic replication. During latency, the virus evades the host immune response and only a few gene products are expressed through cellular DNA polymerase. After stimulation, the virus enters the lytic cycle by a reactivation process [13]. In tumor cells, HHV-8 predominantly persists in a latent form. However, lytic replication is believed to play a critical role in tumorigenesis [13]. When cells are infected by HHV-8, one of the viral gene results in the production of viral interleukin-6 (IL-6), which is thought to be an important growth factor for HHV-8-driven neoplasms. The exact mechanism by which HHV-8 promotes tumor formation is an area of active investigation. Clinically, it is common to have viral reactivation when patients have acquired conditions that suppress cytotoxic T lymphocyte response to viral infection [6]. In the case described here, it is likely that chemotherapy and the steroid used for antiemetic premedication resulted in immunosuppression, leading to reactivation of HHV-8 and consequently the development of KS. Although detection of HHV-8 infection in the neoplastic cells is unambiguous in this case, serology could not be used due to technical limitations. If serology was available, it might have been a better way to assess if the patient had been previously infected.
Reactivation of HHV-8 in patients with solid tumors receiving chemotherapy is less well studied clinically than other viruses such as hepatitis B virus (HBV) and cytomegalovirus (CMV) [14-18]. In these studies, risk factors for viral reactivation included cytotoxic chemotherapy, the use of corticosteroids or rituximab, duration of neutropenia, and the viral load [14-16]. The type of cancer was also found to influence viral reactivation. Among solid tumors, HBV carriers with lymphoma had the highest risk for HBV reactivation while patients with lung, gastrointestinal or breast cancer were at half or one-third the risk of reactivation [19]. Whether this is also true for HHV-8 reactivation, remains to be investigated. Interestingly, similar disease group patients such as those with lymphoma and breast cancer have also been reported to have a greater risk of developing secondary KS [2]. Viral reactivation most frequent followed cessation of chemotherapy, but it could also occur during chemotherapy, between 4 and 36 weeks from treatment initiation, with a median duration of 16 weeks [20]. This observation suggests a common period for reactivation of the DNA virus after the start of chemotherapy and provides a rationale for the introduction of anti-viral agents during treatment.
At present, treatment choices for symptomatic HIV-negative KS include excision, radiotherapy, and anthracycline-containing chemotherapy [21]. Chemotherapy is usually reserved for KS patients with widespread, bulky, or rapidly progressive disease. In the case reported here, radiotherapy was chosen in the beginning due to multi-centric skin lesions on the patient’s left foot and ankle. While the patient was waiting for radiotherapy, the edematous symptoms progressed and a new cutaneous lesion was noted on right lower leg, which indicated the necessity of systemic treatment. Thalidomide, as an anti-angiogenesis agent and immune modulator, has been reported to be of therapeutic benefit in patients with HIV-negative classic KS in small retrospective studies [21, 22]. The rapid remission of skin lesions, within 2 months, in response to thalidomide treatment suggests that the restoration of immunity after cessation of chemotherapy could also play an important role.
Conclusions
In conclusion, our case illustrates that immunosuppressive status during adjuvant chemotherapy can predispose colon cancer patients to HHV-8 associated secondary malignancy KS. Cessation of chemotherapy together with administration of the immunomodulator thalidomide could help control the disease when radiotherapy is unavailable for technical reasons. HHV-8 seropositivity is not uncommon in certain Asian ethnic groups and the population receiving chemotherapy is increasing in line with cancer incidence. As a result, the reactivation of HHV-8 and its related diseases may become more frequent. Further studies are needed to determine the best strategies for monitoring HHV-8 activity and for determining the treatment choice between cytotoxic chemotherapy and experimental drugs.
Grant Support
This work was partly supported by Taipei Medical University /Wan Fang Hospital grants 100TMU-WFH-09.
Conflict of Interest
All authors declare no conflict of interest or financial relationship with a biotechnology manufacturer, a pharmaceutical company, or other commercial entity that has an interest in the subject matter or materials discussed in the manuscript.
| References | ▴Top |
- Iscovich J, Boffetta P, Winkelmann R, Brennan P, Azizi E. Classic Kaposi's sarcoma in Jews living in Israel, 1961-1989: a population-based incidence study. AIDS. 1998;12(15):2067-2072.
doi pubmed - Iscovich J, Boffetta P, Winkelmann R, Brennan P. Classic Kaposi's sarcoma as a second primary neoplasm. Int J Cancer. 1999;80(2):178-182.
doi - Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26(21):3523-3529.
doi pubmed - Morton LM, Curtis RE, Linet MS, Bluhm EC, Tucker MA, Caporaso N, Ries LA, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28(33):4935-4944.
doi pubmed - Farge D. Kaposi's sarcoma in organ transplant recipients. The Collaborative Transplantation Research Group of Ile de France. Eur J Med. 1993;2(6):339-343.
pubmed - Stebbing J, Portsmouth S, Bower M. Insights into the molecular biology and sero-epidemiology of Kaposi's sarcoma. Curr Opin Infect Dis. 2003;16(1):25-31.
doi pubmed - Farge D, Lebbe C, Marjanovic Z, Tuppin P, Mouquet C, Peraldi MN, Lang P, et al. Human herpes virus-8 and other risk factors for Kaposi's sarcoma in kidney transplant recipients. Groupe Cooperatif de Transplantation d' Ile de France (GCIF). Transplantation. 1999;67(9):1236-1242.
doi pubmed - Dukers NH, Rezza G. Human herpesvirus 8 epidemiology: what we do and do not know. AIDS. 2003;17(12):1717-1730.
doi - Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi Sarcoma pathogenesis, and treatment of Kaposi Sarcoma. Cancer Lett. 2011;305(2):150-162.
doi pubmed - Wang YF, Lee SB, Cheng LC, Tai MH, Su IJ. Detection of serum antibodies to three different recombinant antigens of human herpesvirus 8 by immunoblotting: seroprevalence studies in Taiwan. Clin Chim Acta. 2002;320(1-2):37-42.
doi - Huang LM, Huang SY, Chen MY, Chao MF, Lu CY, Tien HF, Lee CY, et al. Geographical differences in human herpesvirus 8 seroepidemiology: a survey of 1,201 individuals in Asia. J Med Virol. 2000;60(3):290-293.
doi - Wang X, He B, Zhang Z, Liu T, Wang H, Li X, Zhang Q, et al. Human herpesvirus-8 in northwestern China: epidemiology and characterization among blood donors. Virol J. 2010;7:62.
doi pubmed - Yu F, Harada JN, Brown HJ, Deng H, Song MJ, Wu TT, Kato-Stankiewicz J, et al. Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog. 2007;3(3):e44.
doi pubmed - Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68(1):105-112.
pubmed - Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, Chan HL, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605-611.
doi pubmed - Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, Lin TH, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology. 2008;47(3):844-853.
doi pubmed - Lim SH, Pathapati S, Langevin J, Hoot A. Severe CMV reactivation and gastritis during treatment of follicular lymphoma with bendamustine. Ann Hematol. 2012;91(4):643-644.
doi pubmed - Kalil AC, Florescu DF. Is cytomegalovirus reactivation increasing the mortality of patients with severe sepsis? Crit Care. 2011;15(2):138.
doi pubmed - Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, Hui P, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62(3):299-307.
doi - Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125(6):1742-1749.
doi pubmed - Rubegni P, Sbano P, De Aloe G, Flori ML, Fimiani M. Thalidomide in the treatment of Kaposi's sarcoma. Dermatology. 2007;215(3):240-244.
doi pubmed - Ben M'barek L, Fardet L, Mebazaa A, Thervet E, Biet I, Kerob D, Morel P, et al. A retrospective analysis of thalidomide therapy in non-HIV-related Kaposi's sarcoma. Dermatology. 2007;215(3):202-205.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


