| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 2, Number 2, April 2011, pages 71-75
The Highest Ever Reported Level of Carbohydrate Antigen 19-9: A Case Report
Hamoud Al-Khallafa, e, Phillip J Monaghanb, Ahmed Ourfalic, Mohamed Elsammakc, Cherrilyn Ambrosioc, Ravinder Sodid, Abduljaleel Poovathumkadavilc
aP.O. Box 15215 (MBC 035), King Fahad Specialist Hospital, Dammam 31444, Saudi Arabia
bDepartment of Clinical Biochemistry, The Christie NHS Foundation Trust, Wilmslow Road, Withington, Manchester, M20 4BX, UK
cP.O. Box 15215, King Fahad Specialist Hospital, Dammam 31444, Saudi Arabia
dDepartment of Clinical Biochemistry and Metabolic Medicine, Royal Liverpool and Broadgreen University Hospital, Liverpool, L7 8XP, UK
eCorresponding author: Hamoud Al-Khallaf
Manuscript accepted for publication February 4, 2011
Short title: Carbohydrate Antigen 19-9
doi: https://doi.org/10.4021/jmc142w
| Abstract | ▴Top |
We present a case of adenocarcinoma of the tail of the pancreas with liver metastases expressing serum carbohydrate antigen 19-9 level of 19,516,020 U/ml. A review of the literature revealed that a concentration of this magnitude has not previously been reported for this tumor marker.
Keywords: Carbohydrate antigen 19-9; Highest reported level; Pancreatic tumor; Adenocarcinoma
| Introduction | ▴Top |
Initially found in colorectal cancer patients, carbohydrate antigen 19-9 (CA 19-9), discovered by Koprowski et al [1], has also been identified in patients with pancreatic, stomach, hepatocellular cancer, and bile duct cancer. Its use for screening asymptomatic populations has been hampered by a false-positive rate of 15% to 30% in patients with non-neoplastic diseases of the pancreas, liver, and biliary tract. In patients who have pancreatic cancer, the literature indicates that higher levels of CA 19-9 tend to be associated with more advanced disease and that levels correlate with tumor burden. Consequently, CA 19-9 has been utilized as a prognostic marker for patients with all stages of the disease [2]. We present herein a case of adenocarcinoma of the tail of the pancreas with multiple liver metastases that lead to intra-hepatic cholestasis. This patient had an extremely high level of CA 19-9 (19,516,020 U/ml) that has never been reported before.
| Case Report | ▴Top |
A 63-year-old Saudi male with type 2 diabetes mellitus (insulin-controlled) and hypertension on anti-hypertensive medications was admitted with anorexia and weight loss over the previous 4 months. The patient did not complain of abdominal pain and there was no history of smoking or alcoholism. On physical exam, his blood pressure was 127/80 mmHg, the pulse rate was 65/min, temperature was 36.4 °C, and the respiration rate was 20/min. His sclera were deeply icteric, lungs were clear, abdominal examination revealed non tender enlargement of the liver with positive shifting dullness.
Laboratory tests were as follows: Hemoglobin: 10.5 g/dl (13.5 - 17.5), hematocrit: 32.4% (41% - 53%), white blood cell count 32.85 x 109/L (4.5 x 109 - 11 x 109/L) and platelet count: 289 x 109 (150 x 109 - 400 x 109). Blood urea nitrogen: 11.3 mmol/L (2.7 - 7.2), creatinine: 76 µmol/L (71 - 115), sodium: 122 mmol/L (135 - 147), potassium: 5.6 mmol/L (3.5 - 5.1), glucose: 11.2 mmol/L (3.9 - 6.1). Alanine aminotransferase level was 151 U/L (17 - 49), aspartate aminotransferase was 286 U/L (8 - 38), alkaline phosphatase was 1251 U/L (54 - 144), gamma-glutamyl transferase 2188 U/L (15 - 85), total bilirubin was 123.4 µmol/L (4 - 21), and conjugated bilirubin was 113.0 µmol/L (0.8 - 3.3). Abdominal ultrasound examination revealed a 4.0 x 5.0 cm mass in the tail of the pancreas. Multiple hypoechoic solid masses, the largest of which measured 7.0 x 4.0 cm, were detected in the enlarged liver. There was no intra or extra-hepatic duct dilatation and the gallbladder was contracted. Ascitic fluid was also detected.
The levels of tumor markers were as follows: alpha feto protein (AFP): 5.3 ng/ml (1.1 - 8.0), carcinoembryonic antigen (CEA): 25.2 µg/L (0 - 5), and CA 19-9: 19,516,020 U/ml (0 - 37).
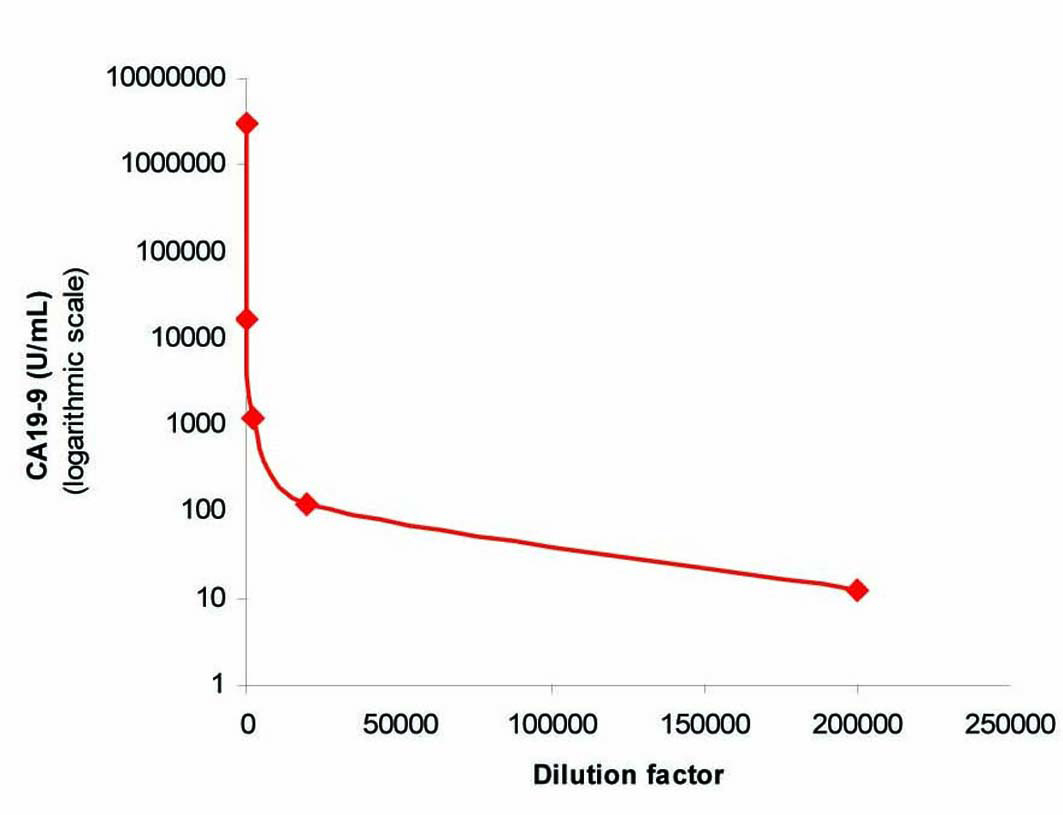
To exclude the possibility of interference as a cause of this extreme CA 19-9 elevation, we performed in our lab a repeat analysis on the same analyzer (Architect i2000), followed by re-analysis of the sample using heterophilic blocking tubes (HBT) with no change in the result. The sample was then sent to a Clinical Biochemistry lab in the UK where the sample was analyzed on a different immunoassay platform (Centaur XP) that gave a comparable result of 3,094,840 U/ml. The sample was curvilinear on serial dilution (Fig. 1), which although did not exclude the presence of interference, did not suggest its presence. Finally, a mean recovery of 102% was obtained with polyethylene glycol (PEG) precipitation (Fig. 2) which excluded the presence of a macro-complex.
 Click for large image | Figure 1. Curvilinear plot obtained by serial dilution of the sample. |
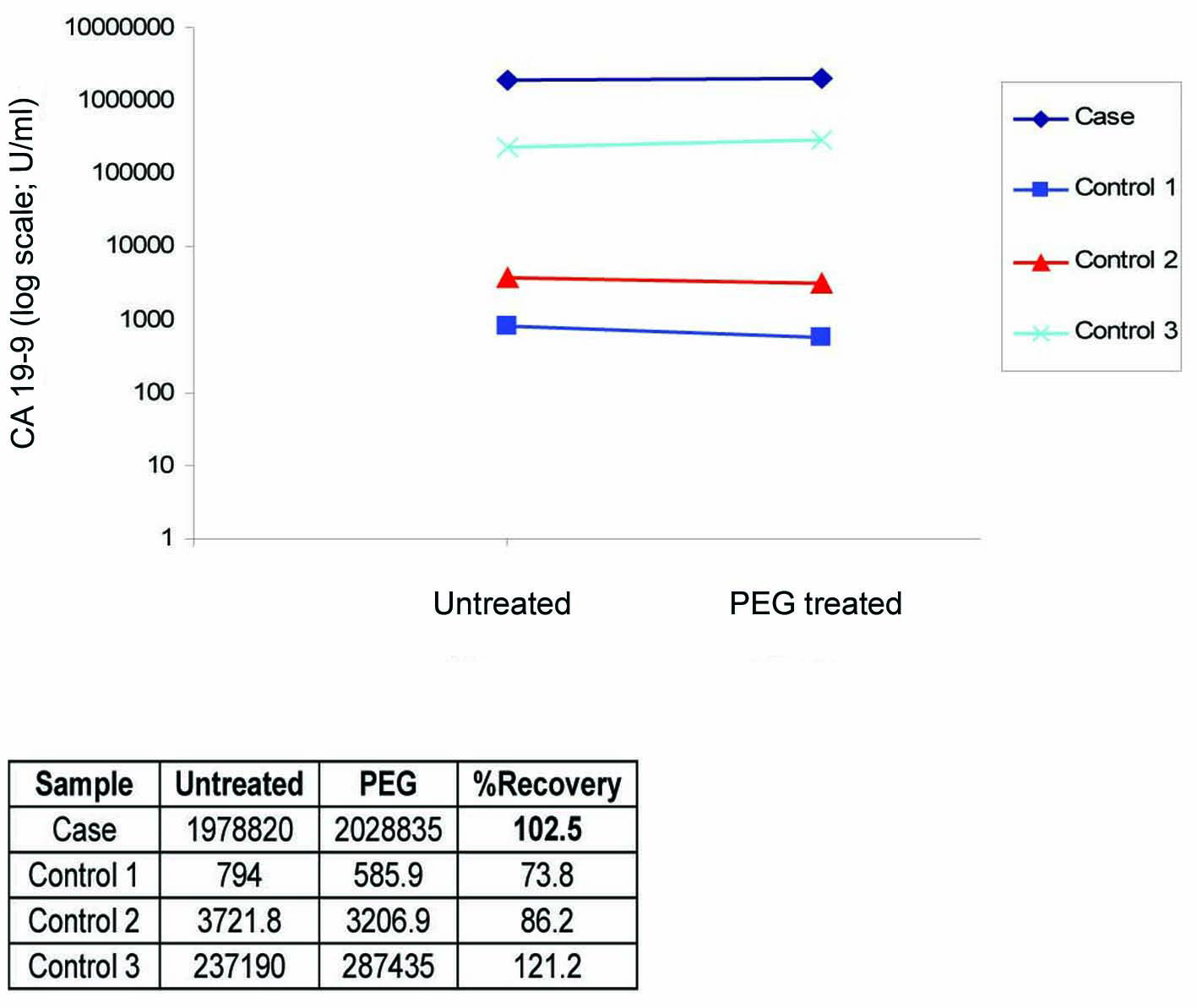 Click for large image | Figure 2. PEG precipitation study showing 102.5% recovery which is consistent with the 3 positive control samples that showed between 74% - 121% recoveries. |
A computerized tomography (CT) scan of chest, abdomen, and pelvis was performed and confirmed the presence of multiple variable size hypo-dense lesions in both liver lobes with no other sign of extra-hepatic metastasis (Fig. 3, 4).
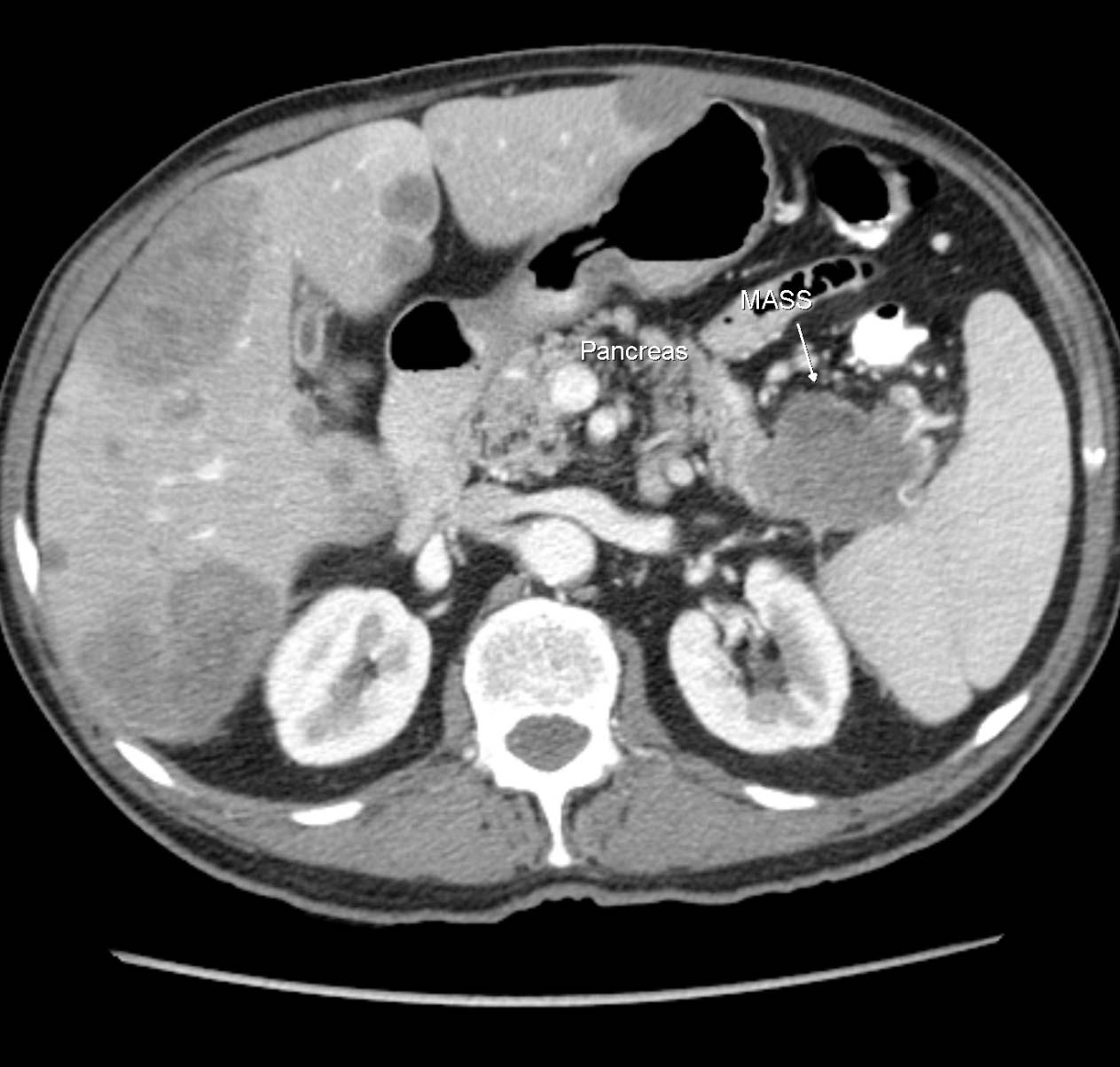 Click for large image | Figure 3. CT Axial showing primary tumor mass (MASS) in the tail of the pancreas and multiple liver metastases. |
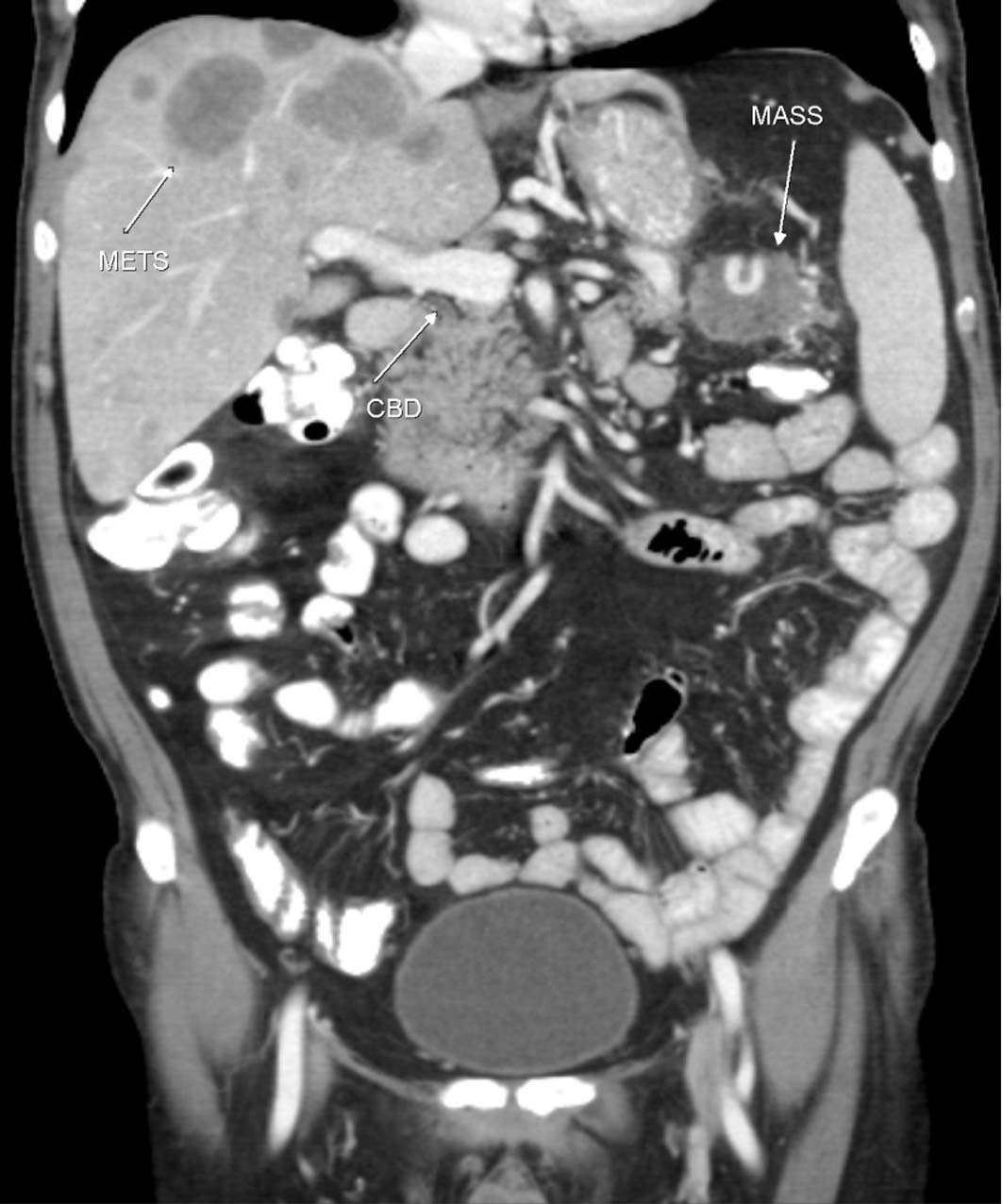
 Click for large image | Figure 4. CT Coronal showing the tumor mass (MASS), liver metastases (METS), and common bile duct (CBD). |
Ultrasound-guided biopsy was taken from the mass in the tail of the pancreas. Histopathological examination revealed moderately differentiated metastatic adenocarcinoma of the pancreas. Before the patient was referred to the Oncology department to determine the therapeutic approach, he developed spontaneous bacterial peritonitis that progressed to sepsis. Post liver biopsy, the patient developed bleeding secondary to fistula between the hepatic artery and portal vein which was embolized by interventional radiology. The patient was transferred to the intensive care unit where he passed away.
| Discussion | ▴Top |
CA 19-9 was initially proposed as a serologic marker for colorectal cancer [3]. Nowadays its use is not recommended for this purpose [3] and CA 19-9 serum concentration is mostly evaluated in patients with suspected pancreatic or biliary cancer, because of its presumed higher specificity in this setting [4]. Unfortunately, CA 19-9 levels have also been reported in other malignancies such as gastric and ovarian cancer and in different benign conditions like cystic fibrosis, hydronephrosis, and Hashimoto’s thyroiditis [5]. Furthermore, elevated CA 19-9 serum concentration can be associated with several conditions producing reduced biliary drainage [6], especially in the presence of cholangitis [7]. It is generally believed that benign conditions cause only moderate elevations, while very high levels are mainly related to malignancies, consistent with the results of previous papers [8, 9]. However, some researchers have published cases that documented extremely elevated CA 19-9 serum concentrations (up to 187,000 U/ml) secondary to benign biliary obstruction that have rapidly normalized after recovery of biliary patency [10].
Production and secretion of CA 19-9 from malignant cells is considered to be responsible for the elevated serum CA 19-9 levels found in malignancy. The reason for the CA 19-9 elevation in acute cholangitis is not clear. Several mechanisms have been postulated, including: 1) leakage of condensed CA 19-9 due to biliary tract obstruction from the bile into blood circulation [4], 2) enhanced CA 19-9 production by irritated bile duct cells exposed to increased biliary pressure [11], 3) enhanced production of CA 19-9 in the bile duct epithelium and the mucosa of the gallbladder induced by the inflammatory process [11], 4) the inflammatory cytokines produced in sepsis due to cholangitis may be a contributing factor [11].
CA 19-9 is measured by immunoassay methodology. It is well documented that immunoassay techniques are prone to interference [12]. Monaghan PJ et al 2009 published an elegant paper [13] documenting a case of elevated CA 19-9 due to the presence of a small molecular weight interferent.
After ruling out analytical interference, we concluded that the extremely high level of CA 19-9 in this patient was produced by two sources. The tumor cells of the primary tumor and liver metastases represent the first source. Secondly, the source which may have had the greatest contribution to CA 19-9 elevation, is the enhanced production of CA 19-9 from the biliary epithelial cells and decreased hepato-biliary clearance because of cholestasis. Cholestasis in this case was caused by the presence of multiple liver metastases and was augmented by the sepsis this patient endured secondary to the spontaneous bacterial peritonitis he had during his admission. Inflammatory cytokines released during sepsis could have stimulated the release of CA 19-9 from biliary epithelial cells and therefore played a part in elevating CA 19-9 level.
In this patient in particular, it is impossible to be certain of the main pathology causing this astonishingly elevated CA 19-9 level due to the multiple pathologies present, each of which is known to cause elevation in CA 19-9 serum concentration. However; since we found no case report with CA 19-9 levels higher than 100.000 U/ml in pancreatic malignancy, while a CA 19-9 level of 187,000 U/ml reported in simple choledocholithiasis [10], we can postulate that the presence of both of these conditions may have had a cumulative effect in producing the extremely high level of CA 19-9 in this patient.
It is clear from this case that CA 19-9 levels can reach several million international units per millilitre. Since knowing the exact level of a tumor marker is important, especially for prognosis and follow up, this case highlights the importance of determining the exact level of this tumor marker rather than just reporting it as “higher than the upper linear limit”. The exact level in this case was more than 16000 times the upper linear limit of our assay (1200 U/ml).
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the journal’s Editor-in-Chief.
Competing Interests
We, the authors, declare that no competing interests exist.
Authors’ Contributions
HK initiated the publication of this case, reviewed the literature and wrote the manuscript. PM undertook most of the analytical procedures pertaining to the sample CA 19-9 level and edited the manuscript. AO in addition to being the clinician in charge of the patient provided the relevant clinical data. MS made significant contribution by his planning and guidance. CA discovered the extremely high level of the tumor marker, brought it to our attention, and performed initial analytical measurements on the sample. RS provided consultation and guidance. AP provided patient’s radiological images. All authors read and approved the final manuscript.
| References | ▴Top |
- Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet 1979;5(6):957-971.
pubmed doi - Katz MH, Moossa AR, Bouvet M. Serologic diagnosis of pancreatic cancer. In CA 19-9 in Neoadjuvant Chemoradiation Pancreatic Cancer. Edited by Von Hoff D, Evans DB, Hruban R. Sudbury, MA: Jones and Bartlett; 2005:235–250.
- Bast RC, Jr., Ravdin P, Hayes DF, Bates S, Fritsche H, Jr., Jessup JM, Kemeny N, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19(6):1865-1878.
pubmed - Kim HJ, Kim MH, Myung SJ, Lim BC, Park ET, Yoo KS, Seo DW, et al. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol 1999;94(7):1941-1946.
pubmed doi - Parra JL, Kaplan S, Barkin JS. Elevated CA 19-9 caused by Hashimoto’s thyroiditis: review of the benign causes of increased CA 19-9 level. Dig Dis Sci 2005;50(4):694-695.
pubmed doi - Katsanos KH, Kitsanou M, Christodoulou DK, Tsianos EV. High CA 19-9 levels in benign biliary tract diseases. Report of four cases and review of the literature. Eur J Intern Med 2002;13(2):132-135.
pubmed doi - Albert MB, Steinberg WM, Henry JP. Elevated serum levels of tumor marker CA19-9 in acute cholangitis. Dig Dis Sci 1988;33(10):1223-1225.
pubmed doi - Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol 2000;95(1):204-207.
pubmed doi - Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol 2000;26(5):474-479.
pubmed doi - Madonia S, Aragona E, Maisano S, Montalbano L, Olivo M, Rossi F, Restivo G, et al. CA 19-9 to rule out pancreatic or biliary cancer among patients with cholestasis: an unsuitable test? Dig Dis Sci 2007;52(4):1125-1127.
pubmed doi - Marcouizos G, Ignatiadou E, Papanikolaou GE, Ziogas D, Fatouros M. Highly elevated serum levels of CA 19-9 in choledocholithiasis: a case report. Cases J 2009;2:6662.
pubmed - Kricka LJ. Interferences in immunoassay—still a threat. Clin Chem 2000;46(8 Pt 1):1037-1038.
pubmed - Monaghan PJ, Leonard MB, Neithercut WD, Raraty MG, Sodi R. False positive carbohydrate antigen 19-9 (CA19-9) results due to a low-molecular weight interference in an apparently healthy male. Clin Chim Acta 2009;406(1-2):41-44.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


