| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 8, August 2013, pages 535-539
Basaloid Squamous Cell Carcinoma of the Rectum: A Rare Entity
Anastasios Machairasa, Dimitrios Tsapralisa, Vassilis D. Samarasb, Nikos Economopoulosc, Anestis Charalambopoulosa, Nickolas Machairasa, Alexandros Charalabopoulosa, d, Evangelos P. Misiakosa
a3rd Department of Surgery, Attikon University Hospital, University of Athens School of Medicine, Rimini 1 Street, Chaidari, Athens, Greece
bDepartment of Pathology, Attikon University Hospital, University of Athens School of Medicine, Rimini 1 Street, Chaidari, Athens, Greece
c2nd Department of Radiology, Attikon University Hospital, University of Athens School of Medicine, Rimini 1 Street, Chaidari, Athens, Greece
dCorresponding author: Alexandros Charalabopoulos, Attikon University Hospital, University of Athens School of Medicine, Rimini 1 Street, Chaidari, Athens, 12462, Greece
Manuscript accepted for publication June 6, 2013
Short title: Basaloid Squamous Cell Carcinoma
doi: https://doi.org/10.4021/jmc1386e
| Abstract | ▴Top |
We report a case of a 33-year old woman with a squamous-cell carcinoma of the rectum, of the basaloid subtype, infiltrating the uterus. The pathologic data and imaging findings that document the presence of the primary tumor of the rectum are presented in detail. Basaloid tumor is a histologic subtype of squamous-cell carcinoma arising usually in the anal canal. In this article we present a case of basaloid carcinoma of the rectum outside the anal canal with a bibliographical review of this rare clinical entity.
Keywords: Basaloid; Squamous-cell cancer; Rectum
| Introduction | ▴Top |
Although adenoma and adenocarcinoma constitute the majority of neoplasms of the colon and rectum, many other tumors in this anatomic region have been described [1]. Of these, some represent extraordinarily rare lesions and may, thus, be the source of difficult decision-making in the clinical practice. Pure squamous cell carcinoma (SCC) is a histologic subtype of tumors of epithelial origin that is not uncommon in glandular organs such as lung and pancreas, but accounts for only 0.1 to 0.25 cases per 1,000 colorectal carcinomas [2-5]. After the first case report in 1919 [6], a total of 74 pure SCC of the colorectum and 147 of mixed squamous and adenosquamous cancer cases have been reported in the literature [5, 7-9].
Michelassi et al [10] found synchronous squamous cell carcinoma of the colon present in 3.2% of the collected cases with colon cancer; 10% had either antecedent, synchronous or metachronous adenocarcinoma of the colon.
Basaloid squamous carcinoma (BSC) is a distinctive variant of squamous cancer that typically occurs in the upper aerodigestive tract and the anal canal [11]. Historically, this rare histologic subtype of squamous cancer has been diagnosed in 3 patients with colorectal tumors, none more proximally than the splenic flexure [12]. Herein we present a case of a basaloid carcinoma of the rectum outside the anal canal, with a comprehensive review of the literature and a clarification of the clinicopathologic features of this rare entity.
| Case Report | ▴Top |
A 33-year-old female of Caucasian origin was referred to our hospital for treatment of a pelvic mass. Three months prior hospitalization, the patient started occasionally noticing blood in her stool, as well as alteration in her bowel habits. The patient reported a 2-week history of persistent constipation and fatigue, with an 8 kg weight loss during the last 7 months. Family history included 3 first-degree and 2 second-degree relatives who suffered from adenocarcinoma of the colon.
The abdomen was moderately distended but free of palpable masses, whereas, on digital rectal examination, a large, fixed, and friable mass was noticed. The results of laboratory tests, including full blood count, blood urea nitrogen, electrolytes, creatinine, and liver function tests revealed solely microscopic hypochromic anemia. Carcinoembryonic antigen (CEA), CA 19-9, CA 125, and α-fetoprotein (α-FP) were normal. A flexible sigmoidoscopy confirmed the presence of an exophytic, friable mass, with its lower edge 5 cm above the dentate line, preventing the endoscope for further passage beyond the lesion. The histopathological analysis of the biopsies taken during the endoscopy, showed a poorly differentiated squamous cell carcinoma of the rectum.
Computed tomography (CT) of the abdomen and pelvis (Fig.1) and magnetic resonance imaging (MRI) of the pelvis were performed (Fig. 2). A heterogeneous solid mass arising from the upper and middle third of the rectum and infiltrating the uterus was evident. There were no signs of metastatic disease in the abdomen, and the chest X-ray did not reveal any pathological findings.
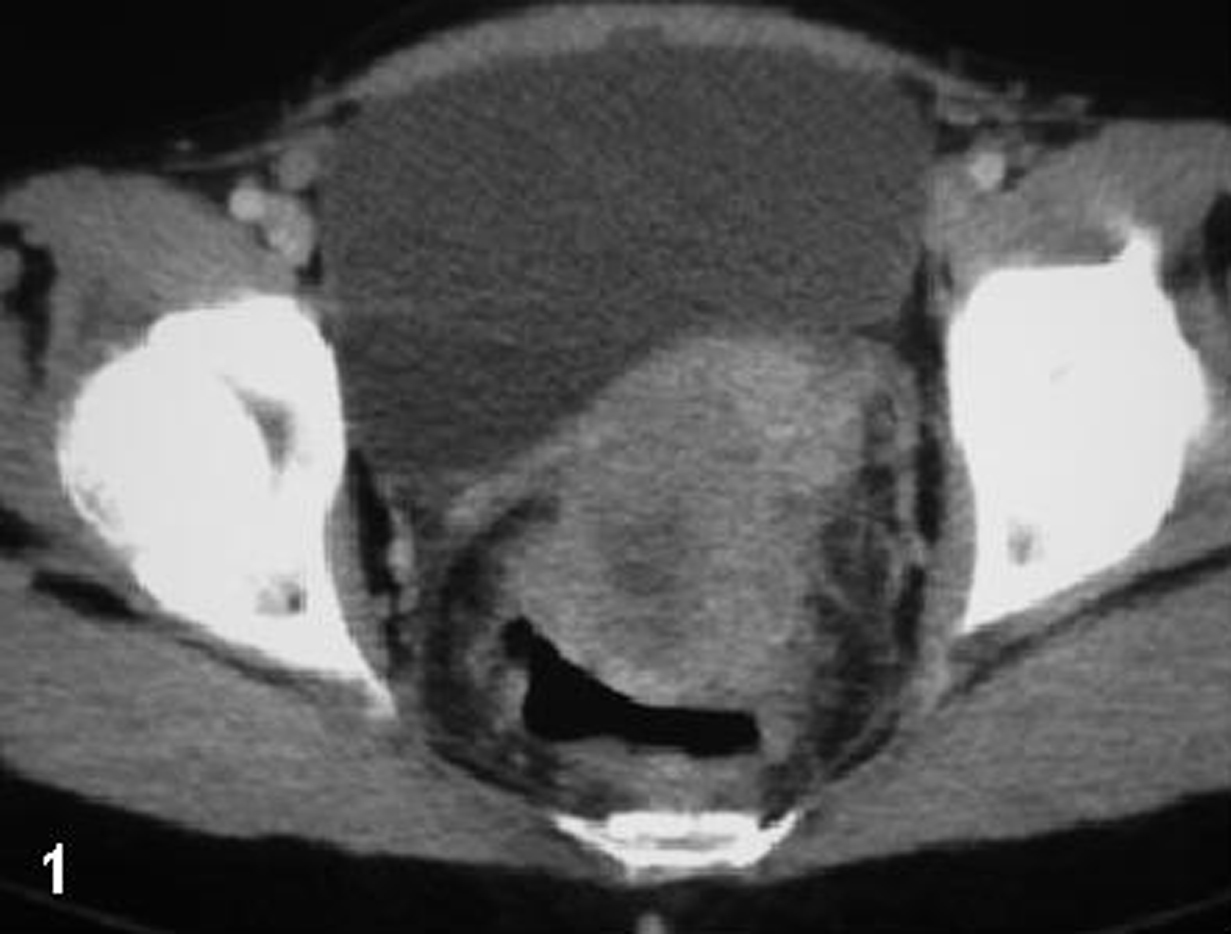 Click for large image | Figure 1. CT of the pelvis: a space occupying lesion originating from the rectal wall invading the uterine corps. |
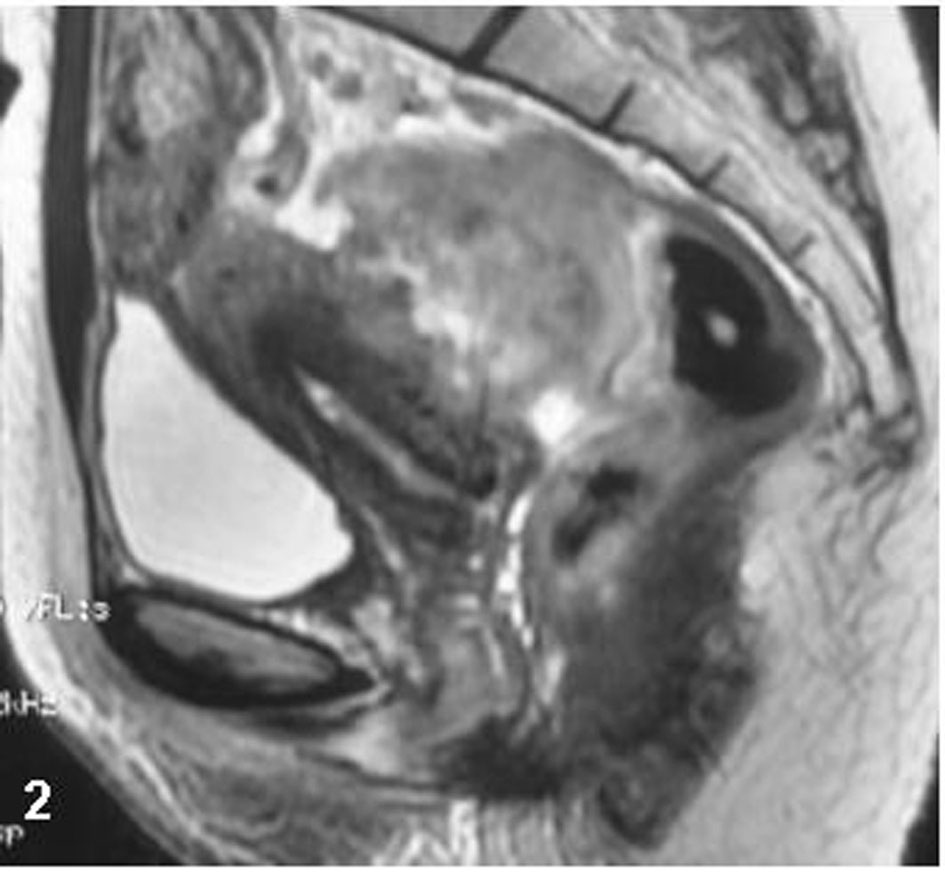 Click for large image | Figure 2. Sagittal MR imaging of the pelvis, T2W sequence: an inhomogeneous massive lesion originating from the anterior rectal wall invades the myometrium. |
At surgery the patient underwent an ultra-low anterior resection with en-bloc resection of the uterus (Fig. 3). The continuity of the gastrointestinal tract was restored with an end-to-end colo-anal anastomosis. Histopathology of the specimen revealed a protruding submucosal neoplasm, of 5 cm in maximum diameter, located in the rectum. The tumour had an extensive central necrosis, whereas a region of tumour rupture within the bowel lumen was identified. Microscopic evaluation documented the presence of a poorly differentiated basaloid squamous-cell carcinoma (Fig. 4) admixed with areas of undifferentiated carcinoma (Fig. 5). In the more differentiated areas, the neoplasm was characterized by the formation of tumor nests with central necrotic debris and peripheral palisading of small to medium sized malignant cells (Fig. 4). The most differentiated tumor areas featured by immunoreactivity to CK34BE12 (Fig. 5), EMA, BerEP4, and NSE, while simultaneous expression of CK18 (Fig. 6) and Vimentin was principally found in the undifferentiated regions. Additionally to large necrotic regions, the neoplasm showed moderate desmoplastic and mild chronic inflammatory stromal reaction. Neoplastic nests invaded the wall of the rectum localized, focally, in the close proximity of the rectum’s serosal surface. Seven (7) out of twenty (20) regional lymph nodes and a mesenteric lymph node were found to be infiltrated by malignant cells. The postoperative recovery of the patient was uneventful and she was discharged on postoperative day 9.
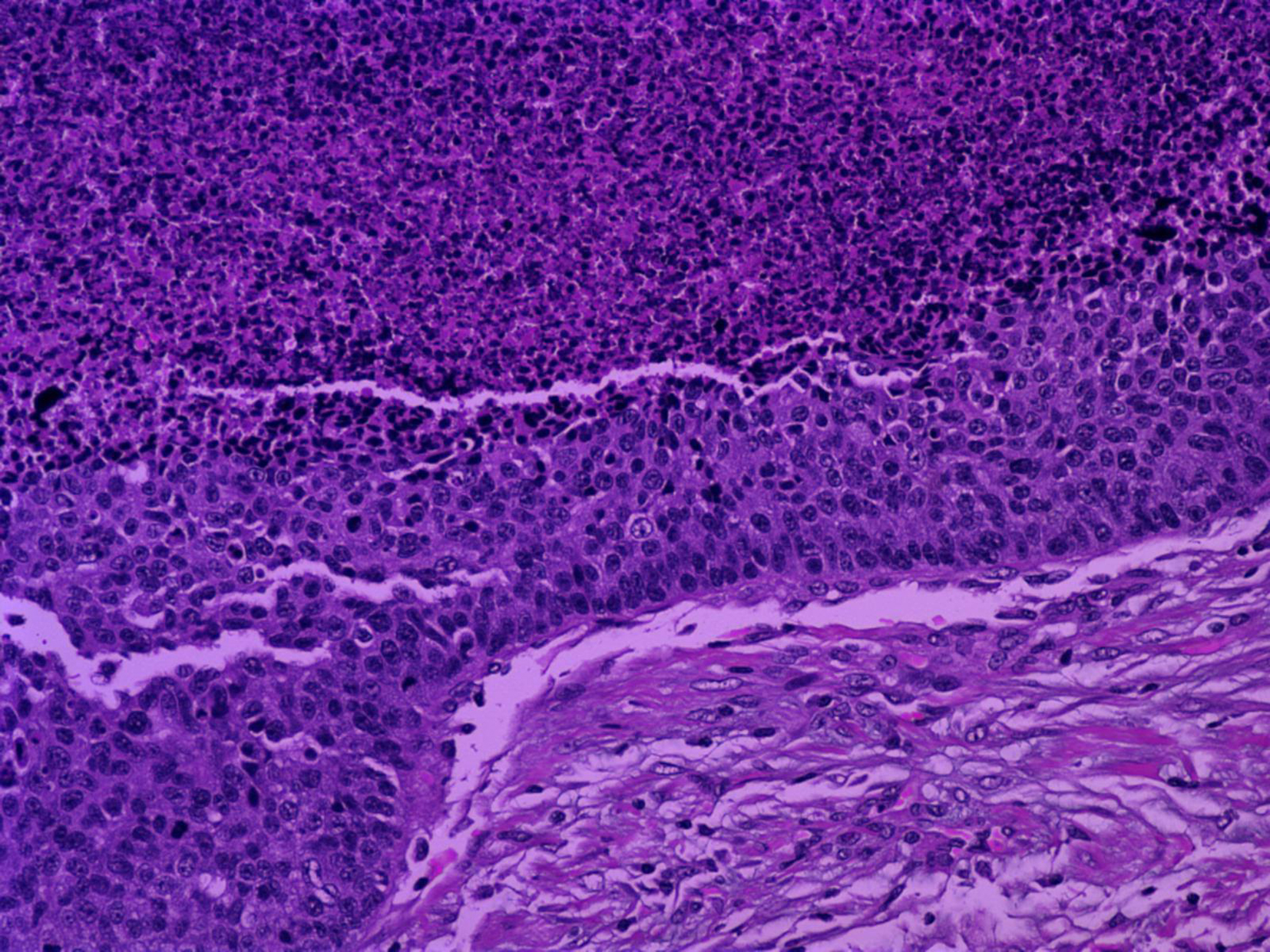 Click for large image | Figure 3. (Hematoxylin and Eosin/X20): Neoplasm in the better differentiated foci characterized by the formation of tumor nests with central necrosis and peripheral palisading of small to medium sized malignant cells. |
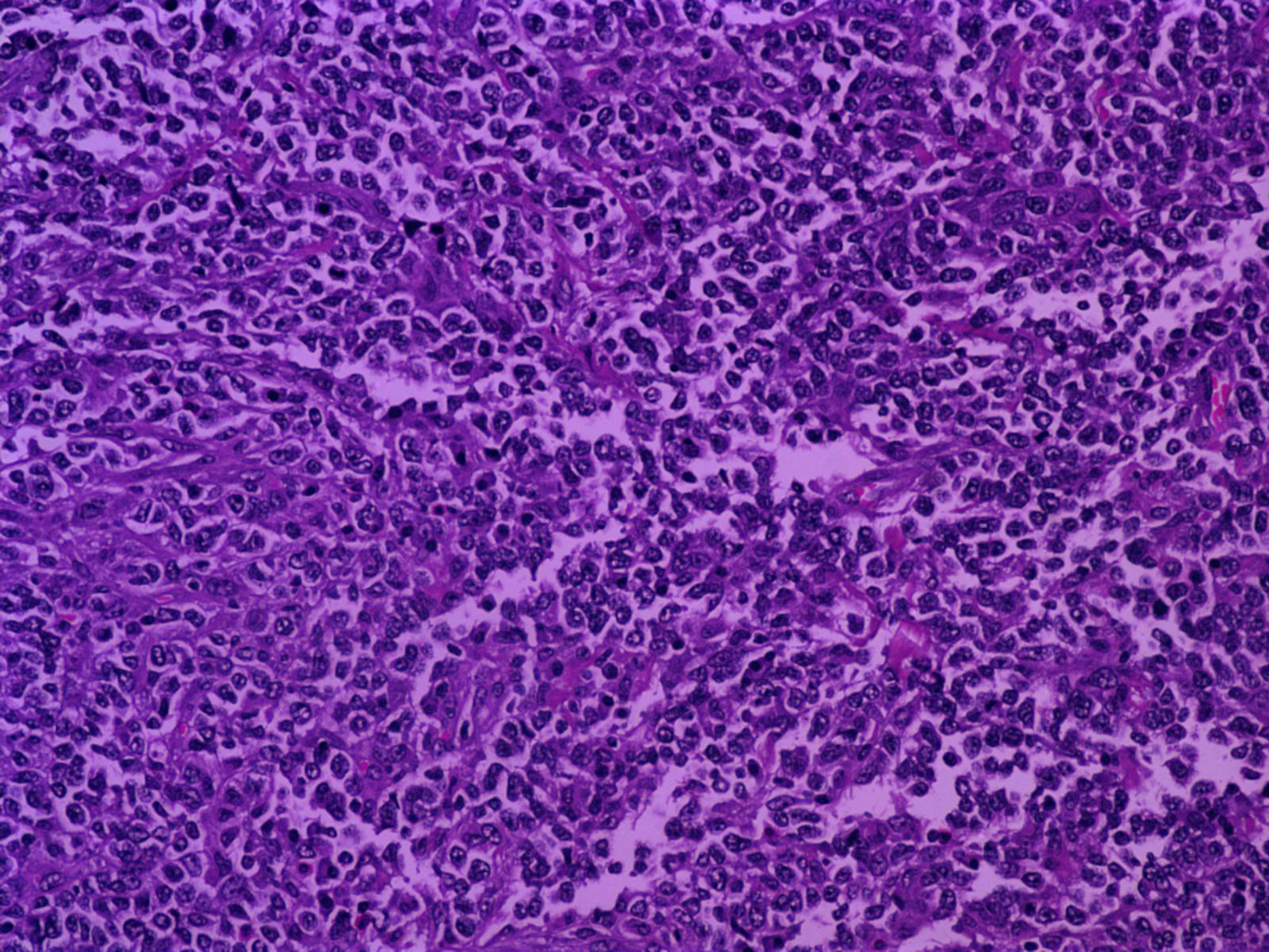 Click for large image | Figure 4. (Hematoxylin and Eosin/X20): Undifferentiated areas within tumor, characterized by diffusely distributed malignant cells. |
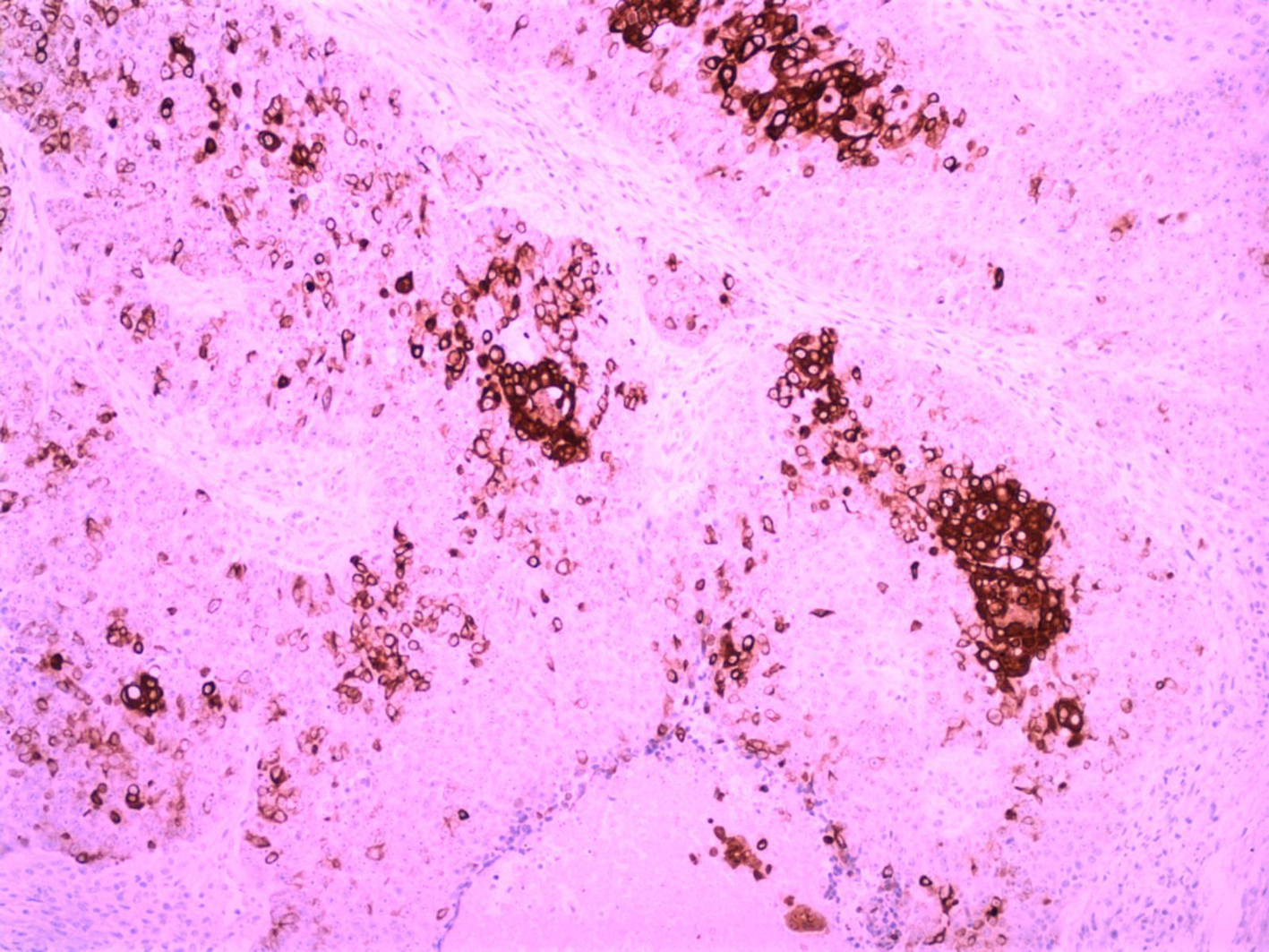 Click for large image | Figure 5. (immunolabeling for CK34BE12/X10): Immunoreactivity to CK34BE12 from the better differentiated neoplastic cells. |
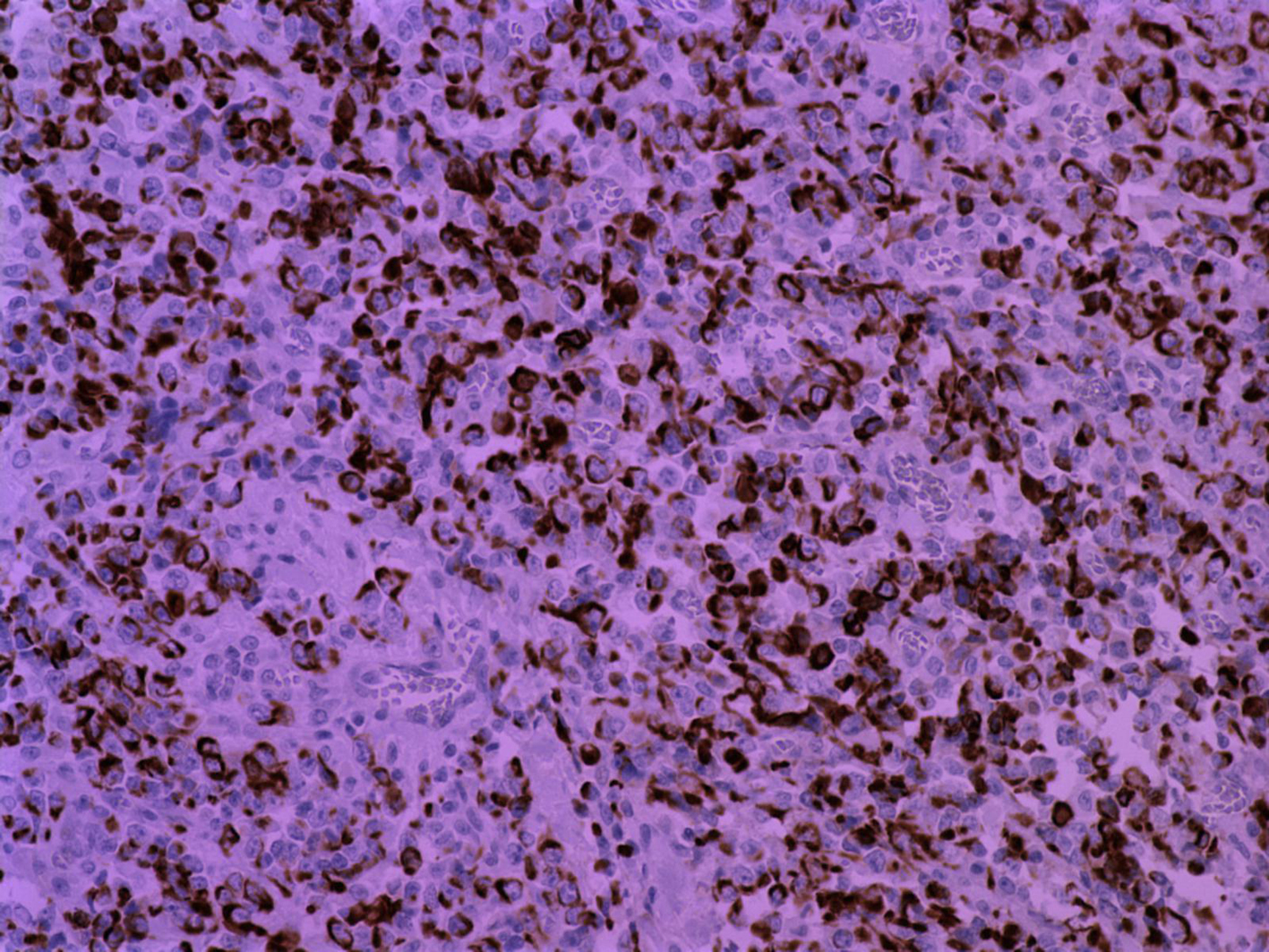 Click for large image | Figure 6. (immunolabeling for CK18/ X20): CK18 expression from undifferentiated malignant cells. |
The patient was given adjuvant chemo-radiotherapy, which included 45 Gy of pelvic irradiation and intravenous administration of 5-fluorouracil (5-FU) and mitomycin C, according to Nigro protocol.
The patient was followed three monthly for the first postoperative year with physical examination and CEA value, which were normal. At 6 months post surgery a full colonoscopy was performed which showed normal colonic mucosa and a healthy anastomosis. Following colonoscopy, radiological imaging of the chest, abdomen and pelvis was scheduled, but the patient failed to attend her appointment and rejected another one due to severe depression and denial. One year following surgery she complained of a vague abdominal pain at the right hypochondrium. An urgent abdominal CT scan revealed a large metastatic lesion of the right hepatic lobe (Fig. 7). The patient was offered an extended right hepatectomy, but before reaching a decision if to undergo second surgery, she died in the meantime of massive pulmonary embolism.
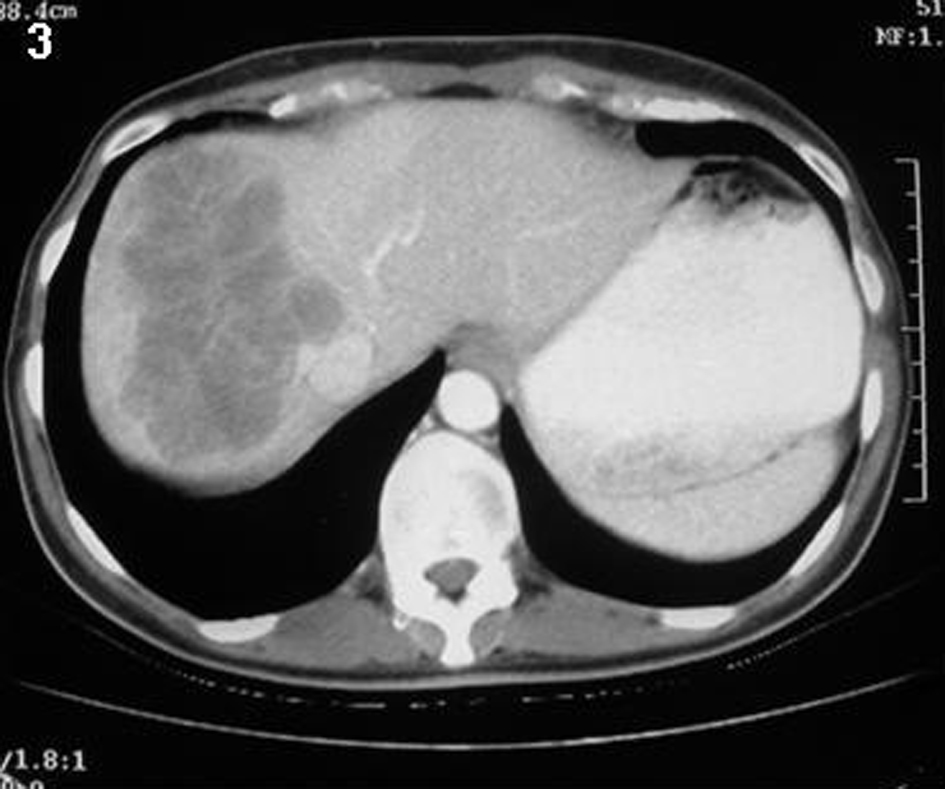 Click for large image | Figure 7. An abdominal CT revealed a large metastatic lesion of the right lobe of the liver. |
| Discussion | ▴Top |
SCC of the colon and rectum is an extremely rare clinical entity. In Japan, Murakami et al described the first case of a pure SCC of the colon in 1974 [13]. It has been suggested that a number of criteria must be satisfied before a diagnosis of primary squamous cell carcinoma of the large bowel is entertained [3, 14, 15]: (1) there must be no evidence in any other organ of a squamous cell carcinoma that might spread directly into the lower bowel or provide a source of intestinal metastases, (2) the affected bowel should not be involved in a fistulous tract lined with squamous cells (colocutaneous fistulas have been described in association with squamous cell cancer), (3) when squamous cell carcinoma occurs in the rectum, care must be taken to exclude origin from the anal canal (that is, there should be a lack of continuity between the lesion and the anal canal epithelium), and (4) SCC must be confirmed by histological analysis. Our case satisfied all the above criteria.
The anal transitional or cloacogenic zone begins approximately 6 to 12 mm above the dentate line and terminates at the junction of columnar rectal mucosa and nonkeratinizing squamous epithelium [16]. The mucosa of the anal transitional zone has an appearance that is intermediate between nonkeratinizing and columnar epithelium, and can contain several cell types: basaloid, columnar, cuboidal, and transitional (urothelium-like), and squamous cells [16, 17]. Classically, tumors arising above the dentate line are mainly nonkeratinizing squamous carcinomas, whereas those below the dentate line are of the keratinizing variety. Therefore it is critical to understand the peculiarities of that anatomic region and to discriminate the origin of the tumor, especially regarding tumors of the lower third of the rectum, before considering such a tumor as squamous cell carcinoma of the rectum and not the anus, as is the norm.
Several mechanisms have been proposed for the pathogenesis of the squamous carcinoma, including proliferation of uncommitted reserve or basal cells following mucosal injury, and squamous differentiation arising in an adenoma [18, 19]. As regards the basaloid variety of squamous cell carcinoma outside the anal canal, it may arise from cloacogenic embryologic nests, squamous metaplastic epithelium, or totipotential basal cells [12]. In mixed adenosquamous variety, Cerezo and others suggested that all such lesions be carefully evaluated by means of immunoperoxidase stains and/or electron microscopy in order to identify squamous features [20, 21].
Symptoms, investigations, and assessment of patients with the divergent histologic subtypes of squamous cancer of the colon and rectum, including basaloid tumors, are similar to those of colon adenocarcinoma, as is evident in our case [5, 22]. However, the prognosis of patients with colorectal SCC is difficult to establish due to the rarity of these tumors. Comer et al suggested a poorer prognosis for patients with colorectal SCC than adenocarcinoma [2]. Indeed, the colorectal SCC seems to be more frequently locally invasive and more likely to involve regional lymphatics than the adenocarcinomas, probably because of delayed diagnosis [23]. In our case, the tumor was pT4 (invasion of the uterus) with lymph node involvement (pN+) according to the TNM classification.
As for the appropriate treatment, resection of the affected segment is the suggested treatment of all colon cases. The addition of adjuvant chemotherapy, consisting of cisplatin, 5-fluorouracil, and leucovorin or etoposide, is based on the pathologic stage of the disease [22]. For rectal lesions, it has been suggested that multimodality therapy, as described by Nigro, be the first line of treatment and that only when this therapy fails should extensive operative procedures be performed [14, 24, 25]. In our case, although the tumor was already locally advanced, it was feasible to resect the tumor radically without sacrificing the anus; despite the addition of the appropriate multimodality scheme, the patient developed liver secondaries within a year.
Conflict of Interest
None declared.
| References | ▴Top |
- Morson BC, Sobin LH. Histologic typing of intestinal tumors: WHO technical report 15, Geneva, World Health Organization, 1976.
- Comer TP, Beahrs OH, Dockerty MB. Primary squamous cell carcinoma and adenocanthoma of the colon. Cancer. 1971;28(5):1111-1117.
doi - Williams GT, Blackshaw AJ, Morson BC. Squamous carcinoma of the colorectum and its genesis. J Pathol. 1979;129(3):139-147.
doi pubmed - Gelas T, Peyrat P, Francois Y, Gerard JP, Baulieux J, Gilly FN, Vignal J, et al. Primary squamous-cell carcinoma of the rectum: report of six cases and review of the literature. Dis Colon Rectum. 2002;45(11):1535-1540.
doi pubmed - Schneider TA, 2nd, Birkett DH, Vernava AM, 3rd. Primary adenosquamous and squamous cell carcinoma of the colon and rectum. Int J Colorectal Dis. 1992;7(3):144-147.
doi pubmed - Schmidtmann M. Zur Kenntnis seltener krebsformen. Virch Arch Pathol 1919; 226: 100-118.
doi - Goodfellow PB, Brown SR, Hosie KB, Feeley K. Squamous cell carcinoma of the colon in an asbestos worker. Eur J Surg Oncol. 1999;25(6):632-633.
doi pubmed - DiSario JA, Burt RW, Kendrick ML, McWhorter WP. Colorectal cancers of rare histologic types compared with adenocarcinomas. Dis Colon Rectum. 1994;37(12):1277-1280.
doi
doi pubmed - Rasheed S, Yap T, Zia A, McDonald PJ, Glynne-Jones R. Chemo-radiotherapy: an alternative to surgery for squamous cell carcinoma of the rectum—report of six patients and literature review. Colorectal Dis. 2009;11(2):191-197.
doi pubmed - Michelassi F, Mishlove LA, Stipa F, Block GE. Squamous-cell carcinoma of the colon. Experience at the University of Chicago, review of the literature, report of two cases. Dis Colon Rectum. 1988;31(3):228-235.
doi pubmed - Chetty R, Serra S, Hsieh E. Basaloid squamous carcinoma of the anal canal with an adenoid cystic pattern: histologic and immunohistochemical reappraisal of an unusual variant. Am J Surg Pathol. 2005;29(12):1668-1672.
doi pubmed - Newell KJ, Penswick JL, Driman DK. Basaloid carcinoma of the colon arising at the splenic flexure. Histopathology. 2001;38(3):232-236.
doi pubmed - Murakami H, Miyagi S, Satoh T. A case of a squamous cell carcinoma of the sigmoid colon. Gera Shinryou (Surgical Diagnosis and Treatment) 1974; 16: 422-425.
- Lafreniere R, Ketcham AS. Primary squamous carcinoma of the rectum. Report of a case and review of the literature. Dis Colon Rectum. 1985;28(12):967-972.
doi pubmed - Miyamoto H, Nishioka M, Kurita N, Honda J, Yoshikawa K, Higashijima J, Miyatani T, et al. Squamous cell carcinoma of the descending colon: report of a case and literature review. Case Rep Gastroenterol. 2007;1(1):77-83.
doi pubmed - Dujovny N, Quiros RM, Saclarides TJ. Anorectal anatomy and embryology. Surg Oncol Clin N Am. 2004;13(2):277-293.
doi pubmed - Takashi H, Masaaki N, Akihiko N, Hiroyuki K. A case of basaloid carcinoma of the anal canal diagnosed as collision tumor with rectal cancer. J Jap Soc Coloproctol 2004; 57: 465-469.
doi - Chevinsky AH, Berelowitz M, Hoover HC, Jr. Adenosquamous carcinoma of the colon presenting with hypercalcemia. Cancer. 1987;60(5):1111-1116.
doi - Vezeridis MP, Herrera LO, Lopez GE, Ledesma EJ, Mittleman A. Squamous-cell carcinoma of the colon and rectum. Dis Colon Rectum. 1983;26(3):188-191.
doi pubmed - Cerezo L, Alvarez M, Edwards O, Price G. Adenosquamous carcinoma of the colon. Dis Colon Rectum. 1985;28(8):597-603.
doi pubmed - Kontozoglou TE, Moyana TN. Adenosquamous carcinoma of the colon—an immunocytochemical and ultrastructural study. Report of two cases and review of the literature. Dis Colon Rectum. 1989;32(8):716-721.
doi pubmed - Juturi JV, Francis B, Koontz PW, Wilkes JD. Squamous-cell carcinoma of the colon responsive to combination chemotherapy: report of two cases and review of the literature. Dis Colon Rectum. 1999;42(1):102-109.
doi pubmed - Copur S, Ledakis P, Novinski D, Mleczko KL, Frankforter S, Bolton M, Fruehling RM, et al. Squamous cell carcinoma of the colon with an elevated serum squamous cell carcinoma antigen responding to combination chemotherapy. Clin Colorectal Cancer. 2001;1(1):55-58.
doi pubmed - Brammer RD, Taniere P, Radley S. Metachronous squamous-cell carcinoma of the colon and treatment of rectal squamous carcinoma with chemoradiotherapy. Colorectal Dis. 2009;11(2):219-220.
doi pubmed - Clark J, Cleator S, Goldin R, Lowdell C, Darzi A, Ziprin P. Treatment of primary rectal squamous cell carcinoma by primary chemoradiotherapy: should surgery still be considered a standard of care? Eur J Cancer. 2008;44(16):2340-2343.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


