| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 10, October 2013, pages 686-688
Papillary Thyroid Carcinoma Developments After Radioactive Iodine Treatment for Toxic Adenoma: A Case Report
Cevdet Durana, f, Mustafa Caycib, Orkide Kutluc, Baris Sevincd, Omer Karahand, Samil Ecirlic, Ceyhan Ugurluoglue
aDivision of Endocrinology, Department of Internal Medicine, Konya Training and Research Hospital, Meram Yeniyol, Konya, Turkey
bDepartment of Nuclear Medicine, Konya Training and Research Hospital, Meram Yeniyol, Konya, Turkey
cDepartment of Internal Medicine, Konya Training and Research Hospital, Meram Yeniyol, Konya, Turkey
dDepartment of General Surgery, Konya Training and Research Hospital, Meram Yeniyol, Konya, Turkey
eDepartment of Pathology, Konya Training and Research Hospital, Meram Yeniyol, Konya, Turkey
fCorresponding author: Cevdet Duran, Division of Endocrinology, Department Internal Medicine, Konya Training and Research Hospital, Meram Yeniyol, Meram, 42100, Konya, Turkey
Manuscript accepted for publication August 12, 2013
Short title: Thyroid Cancer After Radioiodine Therapy
doi: https://doi.org/10.4021/jmc1459w
| Abstract | ▴Top |
Radioiodine is an important radiopharmaceutical agent in nuclear medicine practice for the treatment of hyperthyroidism and differenciated thyroid cancer. One of the long term side effects of ionising radiation is the possibility of radiation induced malignancy. There are limited reports about malignancy after 131I therapy for thyrotoxicosis. In this report, a case with papillary thyroid cancer after radioactive iodine (RAI) therapy for toxic thyroid nodule is presented. In this case, fine needle aspiration biopsy(FNAB) had not been done before RAI therapy and papillary carcinoma was detected four years later than the 444 MBq RAI therapy. The patient underwent bilateral total thyroidectomy and central neck dissection. After the surgery, the patient underwent 5550 MBq RAI for ablation. Summary, differentiated thyroid cancer can be found incidentally in toxic nodule or can be developed as a consequence of radioiodine therapy. FNAB must be performed for all the patients with hot or cold thyroid nodules before RAI therapy and all the patients taking RAI theraphy should be closely followed in terms of nodule growing and cancer development.
Keywords: Thyroid cancer; 131I therapy; Toxic adenoma
| Introductıon | ▴Top |
The usage of radioactive iodine (RAI) therapy for toxic goitre is increasing worldwide, so this issue is a public health problem [1]. RAI is an important radiopharmaceutical agent in nuclear medicine practice for the treatment of hyperthyroidism and differentiated thyroid cancer [2]. One of the longterm side effects of ionising radiation is the possibility of radiation induced malignancy. In the literature, many reports have been published in terms of radiation induced thyroid malignancy after Chernobyl accident [3, 4]. Likewise, it is reported that thyroid cancer can be developed after 131I therapy for thyrotoxicos [5-9]. It is also reported that 131I therapy for diagnostic purpose does not increase the risk of thyroid malignacy although it is commonly used [10]. Besides, radiation exposure can cause thyroid malignancy, RAI therapy is recommended as the first choice for toxic adenoma by American Thyroid Association (ATA) without performing fine needle aspiration biopsy (FNAB). As there is no reported cancer case that developed from hot nodule, routine FNAB is not recommended by ATA [11, 12]. In this report, a case with papillary thyroid cancer after RAI therapy for toxic thyroid nodule is presented.
| Case Report | ▴Top |
A thirty-four year old female patient was admitted to hospital for toxic adenoma in 2007. At the initial investigation, TSH, FT4, FT3, anti-thyroid peroxidase autoantibody and antithyroglobulin autoantibody (Anti Tg) levels were 0.189 µIU/mL (N:0.35-5.5 µIU/mL), 1.48 ng/dL (N: 0.85 - 1.78 ng/dL), 3.26 pg/mL (N: 157 - 4.71 pg/mL), 133 IU/mL (N: 0 - 35 IU/mL) and 337 IU/mL (N: 0 - 40 IU/mL), respectively. Thyroid ultrasonography (USG) showed a calcified solid nodule with cystic degenarations, in a size of 2 cm × 2.5 cm × 2 cm, at right thyroid lobe and the 99mTc thyroid scintigraphy showed a hyperactive/hot nodule at right thyroid lobe (Fig. 1). After the diagnosis, 444 MBq (12 mci) 131I treatment was administered orally in June, 2007. After RAI therapy, patient remained in euthyroid status.
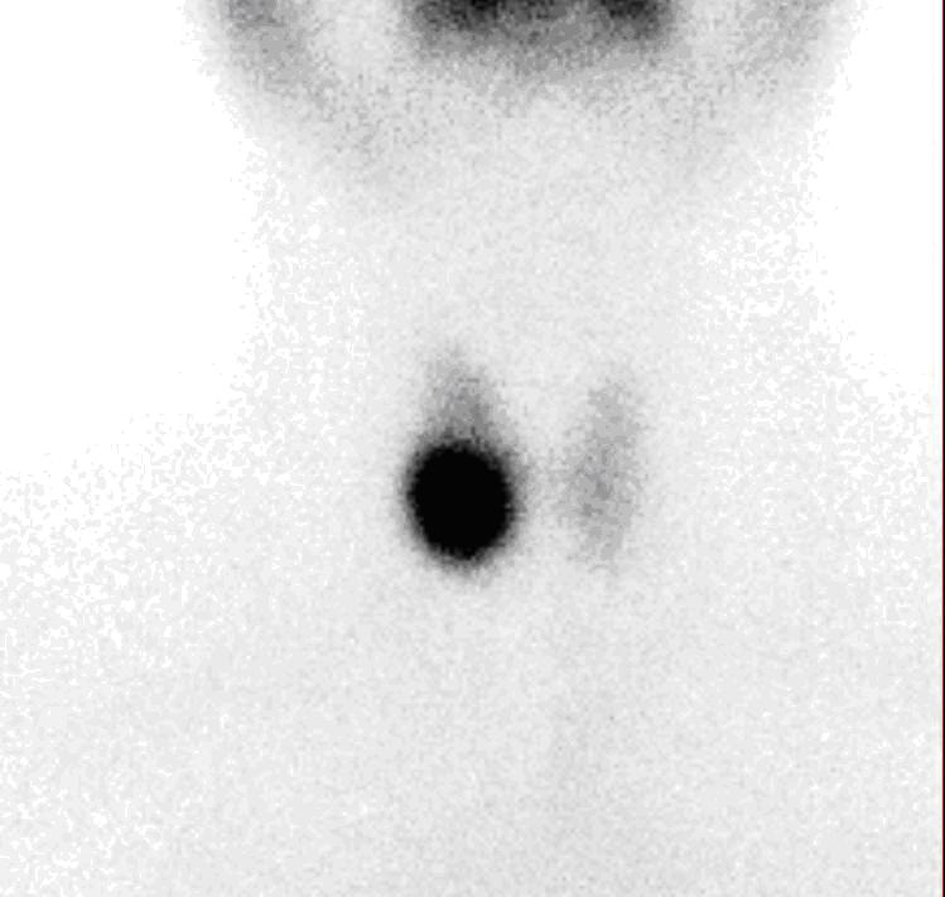 Click for large image | Figure 1. 99mTc scintigraphy showing a hot nodule at the right thyroid region. |
On January 2010, she was admitted at the hospital with a mass at the left side of her neck. After a physical examination, there was a palpable mobile mass at the left side of thyroid lobe. Thyroid USG showed two classified nodules at right thyroid lobe (9.5 and 10 mm in diameter), a heterogeneous mass with cystic areas and lobulated contours (30 mm × 17 mm × 44 mm) at the left side of the cervical region and lymph node with narrow hilus at bilateral cervical regions, larger one measuring 11 mm×5.5 mm. FNAB was performed and it revealed “papillary thyroid carcinoma” in the thyroid nodule at right lobe and “suspected for malignancy” in the mass at the left side.
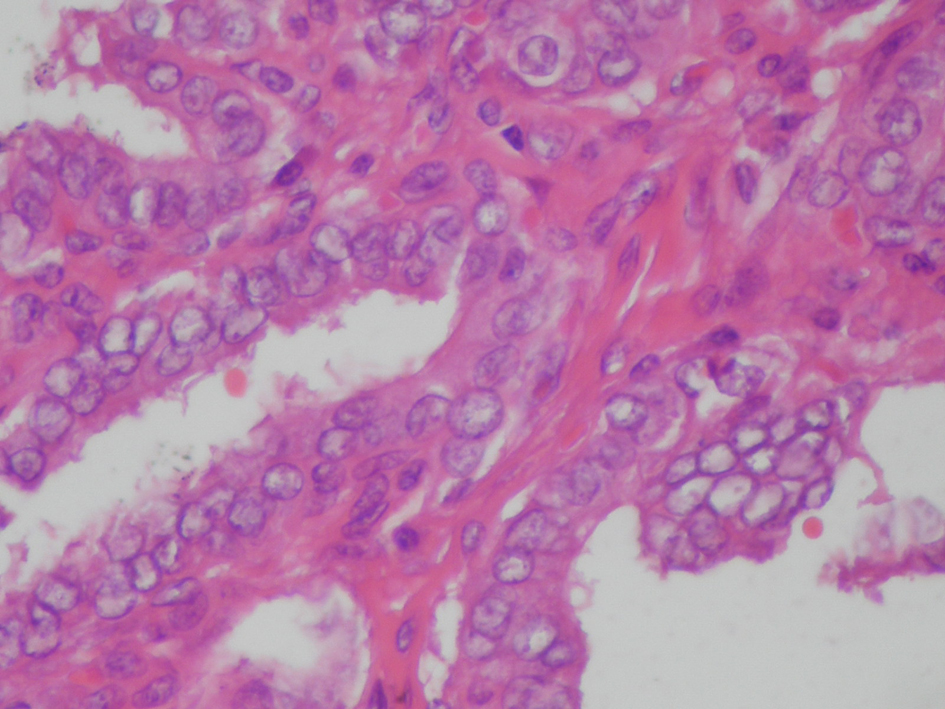
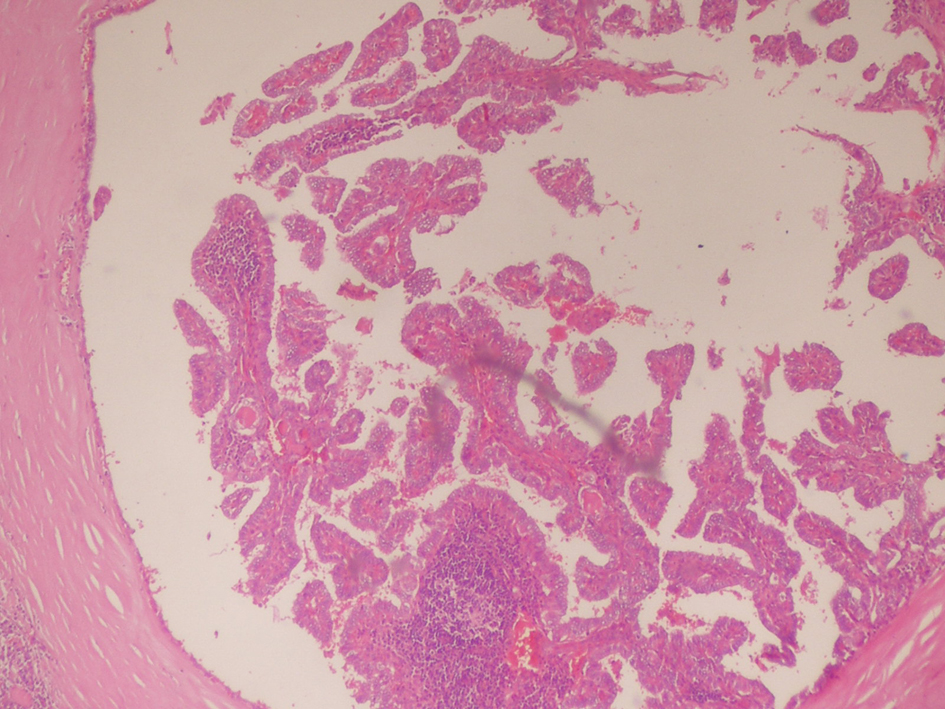
On April 2011, the patient underwent bilateral total thyroidectomy with central lymph node dissection. The histopathological examination showed papillary carcinoma in size 1 cm × 1 cm × 1 cm at right thyroid lobe, 0.4 cm in left thyroid lobe and five metastatic lymph nodes at the left central neck (Fig. 2, 3). On L-thyroxine replacement therapy, post-operative serum TSH, thyroglobulin and anti Tg levels were 0.01 µIU/mL, < 0.2 ng/mL (1.6 - 59.9 ng/mL) and 63.2 IU/mL, respectively.
 Click for large image | Figure 2. Thyroid histopathology showing tumor tissue composed of fibrovasculary core surrounded by papillary formations, partially enclosed by a thick fibrous capsule (Hematoxyline & Eosine, × 80 HP). |
 Click for large image | Figure 3. Thyroid histopathology showing atypical thyrocytes with pale and large nuclei containing glassy intranuclear inclusion bodies, lining the papillary projections. (Hematoxyline & Eosine, × 200 HP). |
On September 2011, the patient underwent 5550 MBq (150 mCi) radioiodine ablation therapy and post therapy, on the 9th day,the whole body scan revealed a remnant at the left thyroid region (Fig. 4).
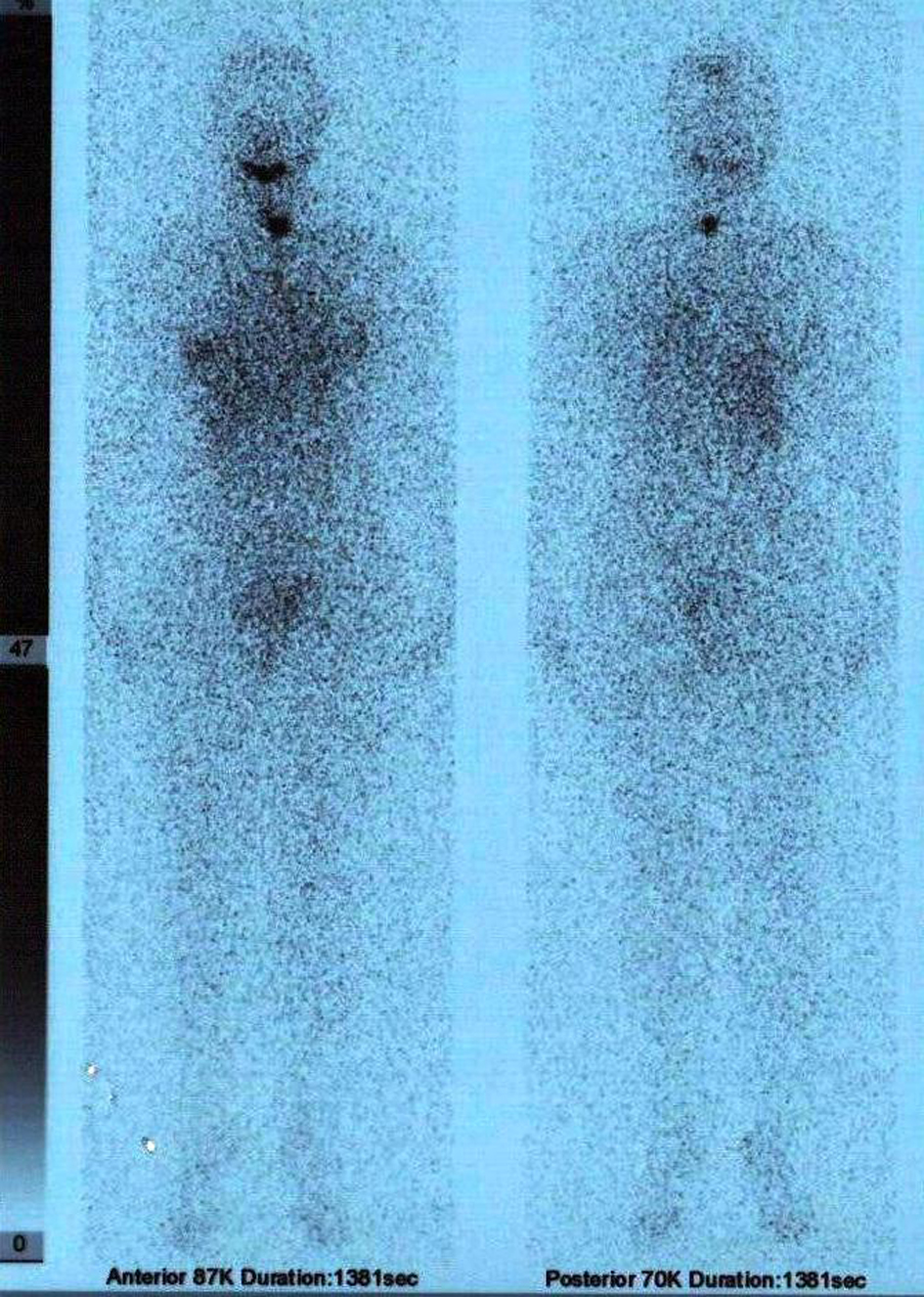 Click for large image | Figure 4. Post ablative 9th day whole body 131I scan showing a remnant at the left thyroid bed. |
| Discussion | ▴Top |
To the best of our knowledge, this is the first case reporting papillary thyroid cancer arising from toxic adenoma following RAI therapy. The prevalence of thyroid nodule increases with age and it is more common in women. Nearly less than 10% of all thyroid nodules are toxic [13]. Initial evaluation of all thyroid nodules require FNAB, however, as the rate of malignancy in toxic nodule is low and benign hyperfunctioning nodules may be cytologically indistinguishable from non functioning benign follicular neoplasms and follicular cancer, ATA doesn’t recommend it for toxic ones [11]. It is reported that toxic nodules are almost never malignant and cold nodules are 5 - 8% malignant [12].
The usage of RAI therapy for toxic goitre is increasing worldwide and the disadvantage of radioactive iodine therapy is the possibility of radiation induced malignancy. 131I has relatively high photon energy (364 keV), long half life (nearly 8 day) and the presence of beta particle emissions [2]. Latency period between radiation exposure and development of thyroid cancer ranges between a minimum of 3 - 7 years and a maximum of 40 - 50 years [3]. The risk of the development of thyroid malignancy decreases with age and after the age of 20 years the risk is less [3, 4]. In our patient, if we accept that the thyroid cancer developed as a consequence of first RAI therapy, there had been nearly 4 years between first RAI therapy and the diagnosis of cancer. This was consistent with the literature for the development of the thyroid cancer after radioiodine.
In the literature, there are few reports about thyroid cancer after RAI therapy for toxic nodulary or diffused goiter. Staffurth et al. reported a folliculary thyroid cancer following RAI therapy for Graves’ disease [5]. In their case, FTC derived 17 years after the RAI therapy. Similarly, there are some reports of anaplastic thyroid cancer after RAI therapy for toxic goiter [6-9].
In the case, FNAB was not performed before the first RAI therapy. It is not known whether the cancer had been there before the therapy or it was developed after the therapy. Therefore, FNAB must be performed for all nodules before RAI therapy.
In conclusion, differentiated thyroid cancer can be found incidentally in toxic nodule or can be developed as a consequence of RAI therapy for toxic nodule. FNAB must be performed for all the patients with hot or cold thyroid nodules before RAI therapy and all the patients taking RAI theraphy should be closely followed in terms of nodule growth and cancer development.
Acknowledgments
Authors have no relevant conflict of to declare.
| References | ▴Top |
- United Nations Scientific Commitee on the effects of Atomic Radiation (UN-SCEAR). Exposure to the public from man-made sources of radiation radiation. (UN-SCEAR) 2000 report to the general assembly, with scientific annexes. http://www.unscear.org/docs/reports/annexc.pdf
- Basic Principles, Radiopharmaceuticals. Ziessman HA, O’malley JP, Thrall JH (Eds). In: Nuclear Medicine. Elsevier Mosby Philadelphia, PA, 2006, Chapter 1, pp. 3-19.
- Papadopoulou F, Efthimiou E. Thyroid cancer after external or internal ionizing irradiation. Hell J Nucl Med. 2009;12(3):266-270.
pubmed - Cardis E, Howe G, Ron E, Bebeshko V, Bogdanova T, Bouville A, Carr Z, et al. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006;26(2):127-140.
doi pubmed - Staffurth JS, Holl-Allen RT. Follicular carcinoma of the thyroid following radioactive iodine treatment for Graves' disease. Postgrad Med J. 1988;64(757):878-880.
doi pubmed - Maatouk J, Barklow TA, Zakaria W, Al-Abbadi MA. Anaplastic thyroid carcinoma arising in long-standing multinodular goiter following radioactive iodine therapy: report of a case diagnosed by fine needle aspiration. Acta Cytol. 2009;53(5):581-583.
doi pubmed - Cannizzaro MA, De Maria A, Fazzi C, Mazzone G, Terminella A, Fiorenza G, Veroux PF. Anaplastic carcinoma of the thyroid: long-term survival. Minerva Chir. 1993;48(21-22):1293-1299.
pubmed - Hayes FJ, Sheahan K, Heffernan A, McKenna TJ. Aggressive thyroid cancer associated with toxic nodular goitre. Eur J Endocrinol. 1996;134(3):366-370.
doi pubmed - Vescini F, Di Gaetano P, Vigna E, Pascoli A, Cacciari M. Anaplastic thyroid carcinoma in a 49 year-old woman with a long-standing goiter. A case report. Minerva Endocrinol. 2000;25(3-4):81-83.
pubmed - Dickman PW, Holm LE, Lundell G, Boice JD, Jr., Hall P. Thyroid cancer risk after thyroid examination with 131I: a population-based cohort study in Sweden. Int J Cancer. 2003;106(4):580-587.
doi pubmed - Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
doi pubmed - Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, Duick DS, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12(1):63-102.
doi pubmed - Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, Braverman LE, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156(19):2165-2172.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


