| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 1, Number 2, October 2010, pages 64-67
Complete Response to Adjuvant Full Dose Photons Megavoltage Radiotherapy of Giant Cell Tumor of the Temporal Bone
Issam Lalyaa, d, lamia Boulaamaneb, Tayeb Kebdania, Khalid Hassounia, Mohamed Elmarjanyc, Khalid Andaloussic, Khalid Hadadic, Hassan Sifatc, Hamid Mansouric, Hassan Errihanib, Noureddine Benjaafara, Brahim Khalil Elgueddaria
aDepartment of Radiotherapy, National Institute of Oncology, Rabat, Morocco
bDepartment of Medical Oncology, National Institute of Oncology, Rabat, Morocco
cDepartment of Radiotherapy, Military hospital MED V, Rabat, Morocco
dCorresponding author: Foyer d’internat Hospital Avicenne, Rabat, Morocco
Manuscript accepted for publication September 8, 2010
Short title: Photons Megavoltage Radiotherapy
doi: https://doi.org/10.4021/jmc15w
| Abstract | ▴Top |
Giant cell tumors (GCTs) of the skull bones are extremely rare and can be very aggressive. The tumor location and the frequent associated local infiltration did not allow adequate surgical treatment. However, with modern microsurgery techniques, a more complete removal of tumor may be possible. Nevertheless, postoperative radiation therapy appears to be the most effective tool in achieving local control of the tumor. We report a 30-year-old young male, treated initially for a GCT of the right temporal bone with surgery alone, which presented nine months later. A local recurrence necessitated surgery and adjuvant radiotherapy using high energy photons without mixing with protons. The patient achieved a good outcome and local control for more than 12 months of follow-up, with good cosmetic result and without significant toxicity. The aim of our work is to demonstrate that 3D conformal radiation therapy using only photons of high energy is a safe and effective adjuvant treatment of GCT of the temporal bone, and therefore equivalent to proton therapy.
Keywords: Giant cell tumor; Skull bone; Temporal bone; Surgery; Adjuvant radiotherapy; Photons; Protons; Complete response
| Introduction | ▴Top |
Giant cell tumors (GCTs) of bone are uncommon primary bone neoplasms that usually occur in the epiphysis of long bones. When these lesions are encountered in the skull, which is an extremely rare site of development of these tumors, they are preferentially seen to involve the sphenoid, and temporal bones. GCTs are benign neoplasms but can be locally aggressive, especially with local recurrence after surgery. A tendency towards late malignant change with metastases especially to the lung has been reported. We report a clinical case of GCT of the right temporal bone in a 30-year-old young man. The patient received initial treatment based on surgery alone, with a good local control obtained during nine months. The tumor had local recurrence, with adjacent soft tissue infiltrating. The patient then received surgery and adjuvant radiotherapy. He is recurrence free for more than 15 months of follow-up.
| Case Report | ▴Top |
We report a clinical case of 30-year-old young man, with no history of disease, presenting three years ago with right otalgia associated to an ipsilateral temporal mass well visualized on the CT scan . Patient underwent surgery, and pathological examination demonstrated a tumor of myeloplexus. After nine months of a well control, patient had local recurrence with hearing loss clinically, associated to a larger and a consistent mass in the temporal area. CT scan showed in the temporal bone, the presence of multiple lacunar images, associated to a tissue component that invades the subcutaneous soft tissues in outer part and in outside the dure-mere and cerebral parenchyma, reaching the rocher with an extent to the ossicular chain of the middle ear . The patient was lost from view for two years. A new CT scan showed an increase of the tumor size. Then, the patient had surgery; with macroscopic complete resection. Pathological examination showed a giant cell tumor, grade II of Jaffé Lichtenstein. The patient received adjuvant radiotherapy with photons 6 and 18MV, at a dose of 60 Gy (2 Gy per day, five days a week). After more than 15 months of follow-up, the patient achieved local control of complete response.
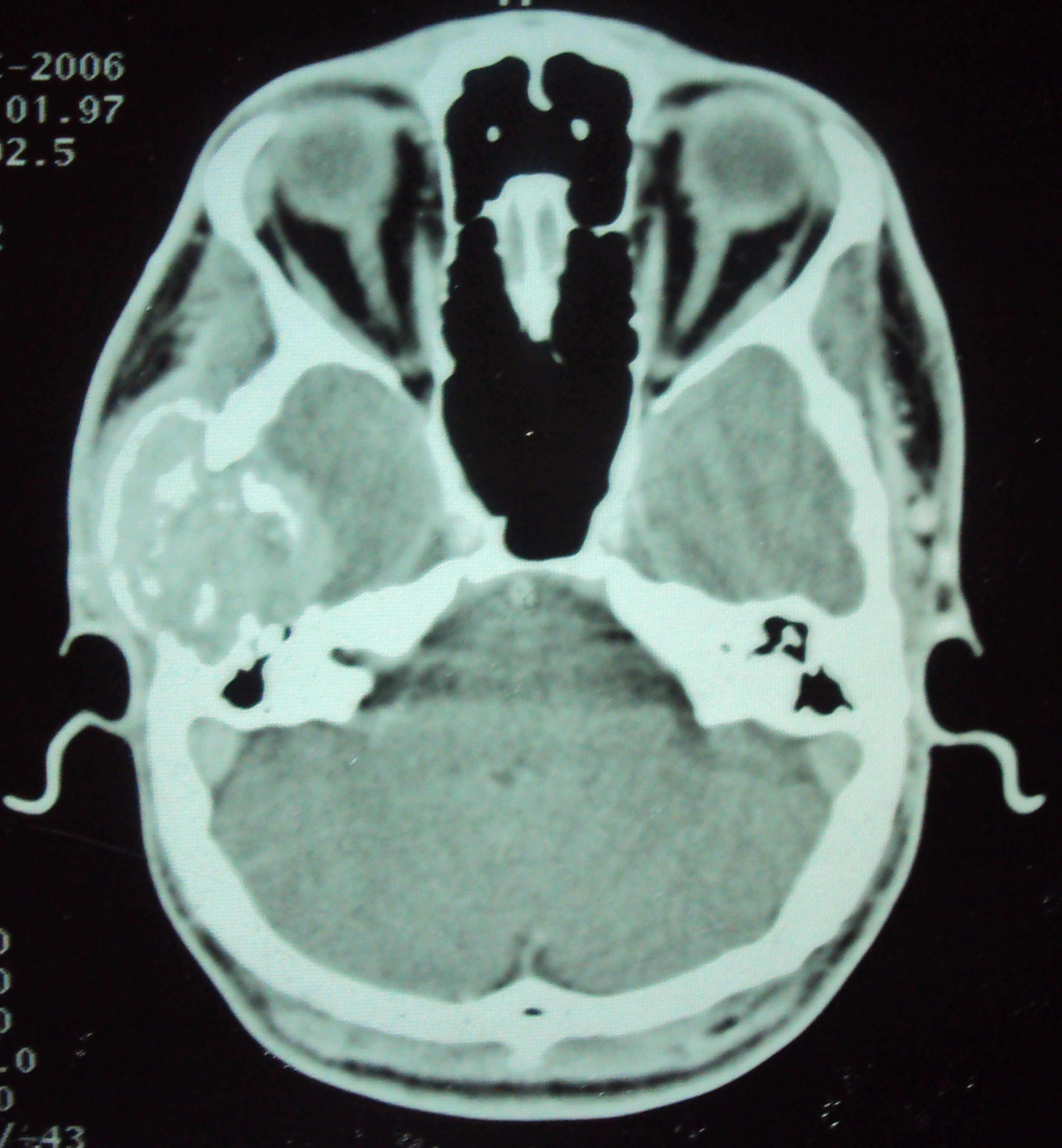 Click for large image | Figure 1. CT SCAN showing a process of the right temporal bone. |
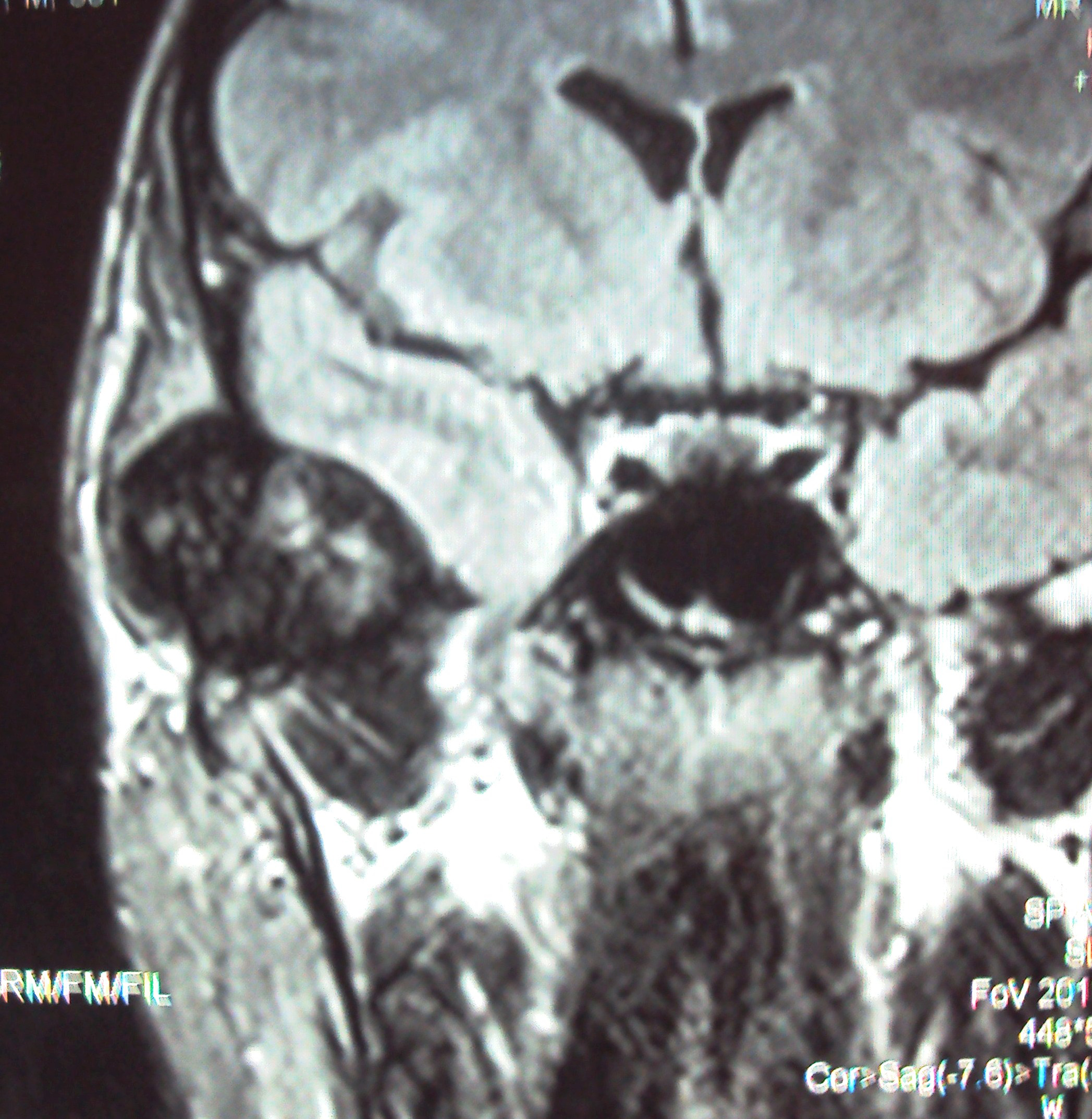 Click for large image | Figure 2. MRI showing a process of the right temporal bone. |
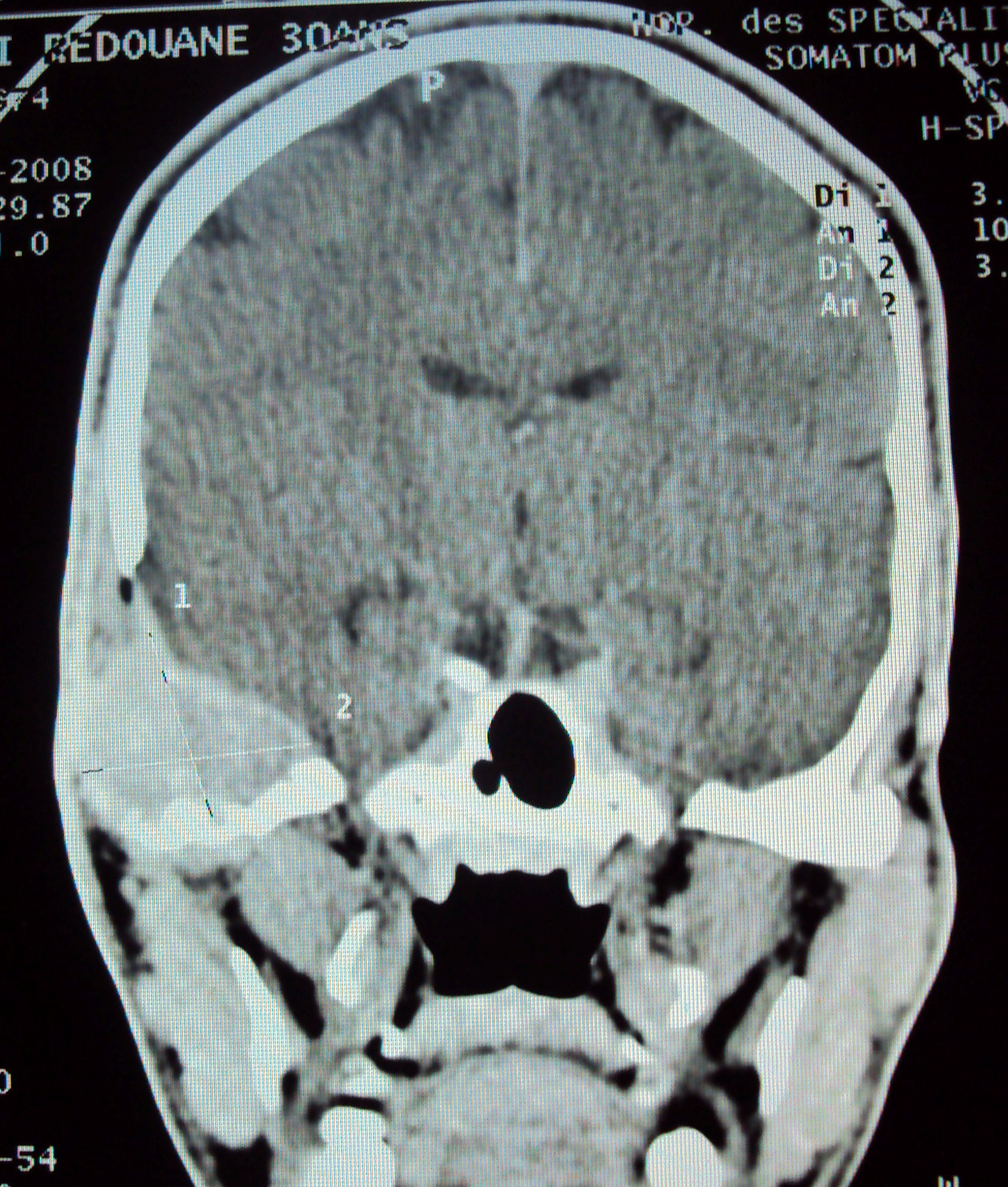 Click for large image | Figure 3. CT SCAN showing recurrence in the same area. |
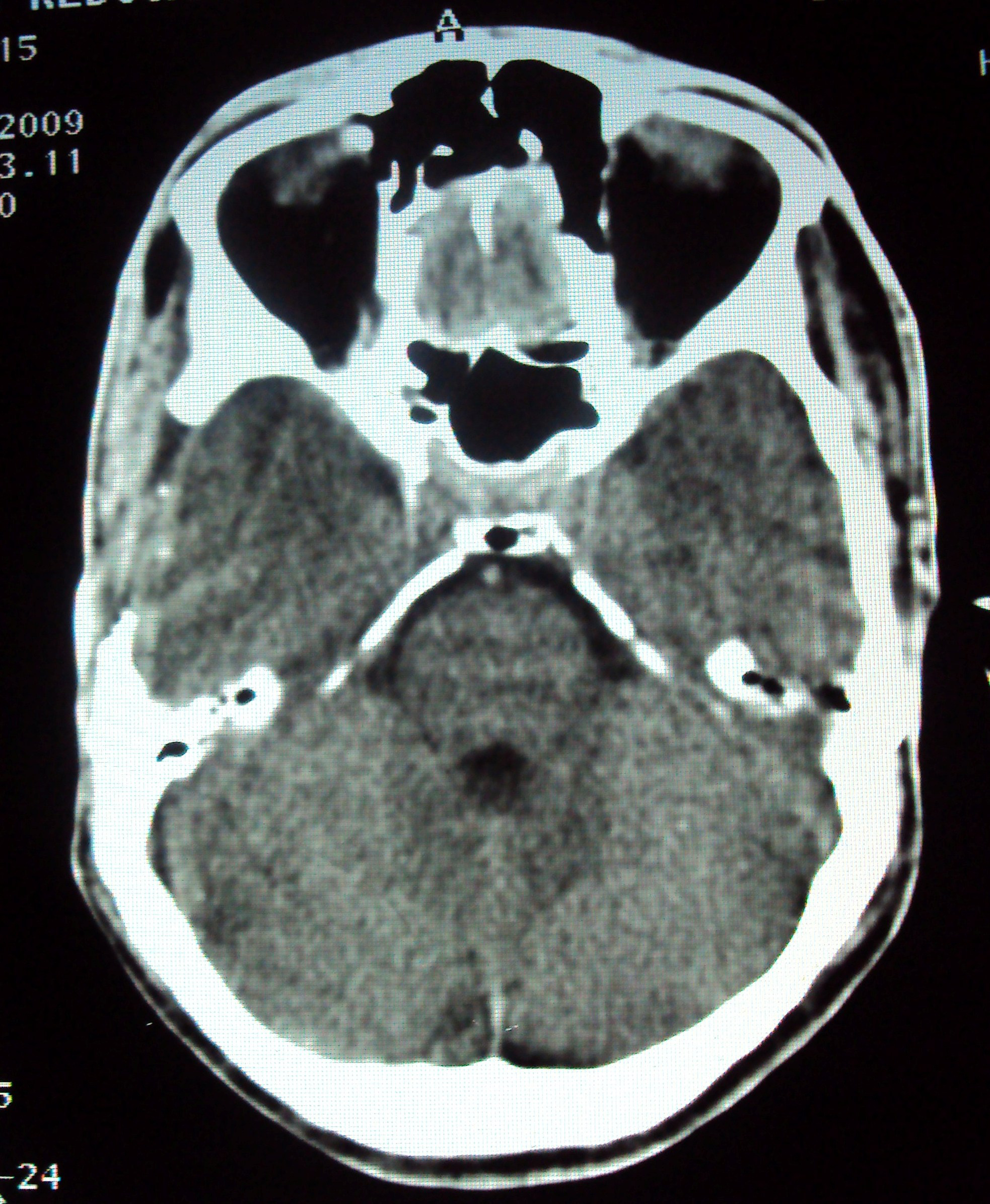 Click for large image | Figure 4. CT SCAN showing complete response after radiotherapy. |
| Discussion | ▴Top |
GCT is a relatively common skeletal tumor, accounting for 4-9.5% of all primary osseous tumors and 18-23% of benign bone neoplasms [1]. Sir Astley Cooper was the first to describe a GCT in 1818. They were previously known as myeloid sarcoma, tumor of myeloplexus, osteoblastoclastoma, or osteoclastoma [2]. Approximately 2% of GCTs present in the head and neck, primarily in the sphenoid, ethmoid, or temporal bone as these sites develop from enchondral ossification [2]. Given the lack of pathognomic radiographic features, a biopsy of a solid (not cystic) component of the lesion is the only definitive way to confirm the diagnosis of GCTs [2]. When they occur in unusual sites, such as the skull, it may be difficult to make a correct preoperative diagnosis. Giant cell tumors are locally aggressive bone lesions characterized by vascularized tissue that contains numerous multinucleated giant cells dispersed through plump, spindly and/or ovoid cells [3]. Jaffe and Lichtenstein had described three levels of aggressiveness:(1) Grade I with absence of atypia and rare mitoses; (2) Grade II, where stromal cells show some atypical characteristics, but insufficient to be classified as malignant tumors, with a sub-grade II+ corresponding to potentially malignant lesions; (3) Grade III reserved for malignant tumors [4]
Otalgia, headache, hearing loss, and cranial nerve palsies are the most common presenting symptoms of temporal bone GCTs. As to ancillary diagnostic tests, skull X rays and angiography have represented the traditional investigations for cranial osteoclastomas [5]. The advent of modern neuroimaging technology has changed the traditional scenario in this as well as generally in all cranial lesions. CT and magnetic resonance imaging (MRI) have maximally restricted the indications for diagnostic arteriography. With a well-defined, expansile bony lesion of significant hypointensity on T2-weighting, bony erosions and no abundant matrix calcifications on CT, one should consider giant cell tumor [6]. Prognosis of GCTs is mainly related to extent of surgical excision, with little contribution from radiographic and histologic grading systems [7]. Treatment options and prognosis are mainly derived from the literature on tumors in long bones [8]. Primary management was recommended by Prosser et al, using curettage for intraosseous giant-cell tumors without adjuvant treatment or filling agents, but tumors with soft tissue extension or with local recurrence require more aggressive treatment [9]. The treatment of choice is complete surgical excision which if achieved can be curative [10]. However, the skull base location of these tumors can make total surgical excision hazardous and not possible [10]. While adjuvant radiotherapy is recommended to eliminate residual tumor tissue, there is evidence that irradiation predisposes the tumor to subsequent sarcomatous degeneration [7]. Therefore, a single course of moderate dose super voltage radiation is recommended in achieving a high success rate and at the same time lowering the likelihood of malignant transformation [10]. A single modality extremity radiation therapy protocol using 40 to 60 Gy over 3 to 6 weeks yielded 85% local control rate, as determined by examination, resolution of pretreatment symptoms, and radiographic studies [11]. Doses less than 30 Gy with prolonged fractionation result in increased local recurrence and are not recommended [12]. With large doses delivered by protons (57.6 to 61.2 Gy), Hug et al reported the control of the disease in four children, all followed for more than three years [13]. The use of protons allow to best protect the healthy tissues adjacent to the tumor and deliver a higher dose which is confined to the target volume [13], but in our case we achieved the same objectives using 3D conformal radiotherapy with high energy photons, the homogeneity of the dose and the protection of healthy tissue were ensured by using wedge filters and a multileaf collimator. For unresectable tumors, radiotherapy remains the only option [14]. Chemotherapy, on the other hand, has not been largely evaluated in the skull base GCTs. Yamamoto et al reported two cases of cranial base GCT that responded well to chemotherapy on periodic CT scans with regression [15], where adriamycin was used after partial tumor excisions. Metastases occur in only 2% of cases and are usually to the lungs, but spread to rare areas like lymph nodes, mediastinum, skin, scalp and pelvis has been reported [16]. However, the rate of malignant transformation in previously irradiated lesions is between 5 and 10% [2].
Although rare, GCT can occur in the skull. Preferential sites for GCT of the skull are the temporal and the sphenoid bone. The location and the infiltrative quality of these tumors can be extremely devastating, leaving the patient severely disabled. Radiotherapy alone or in combination with surgery is a safe and effective treatment for primary and recurrent giant cell tumors of the temporal bone. Radiotherapy should be used in their management if surgery would result in significant functional morbidity, and should be considered in this select site where the probability of recurrence is high and where there is potential for significant morbidity from relapse or subsequent surgery.
Declaration of Competing Interests
All authors declare that they have no competing interests.
| References | ▴Top |
- Bertoni F, Unni KK, Beabout JW, Ebersold MJ. Giant cell tumor of the skull. Cancer 1992;70(5):1124-1132.
pubmed doi - Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics 2001;21(5):1283-1309.
pubmed - Spallone A, Flores GL, Zaldivar LO, Estupinan B. Giant cell tumor (osteoclastoma) of the petrous bone: case report. Skull Base Surg 1999;9(2):155-159.
pubmed doi - Jaffe HL, Lichtenstein L, Portis RB. Giant cell tumor of bone: its pathologic appearance, grading, supposed variants and treatment. Arch Pathol(Chicago) 1940;30:993–1031.
- Dahlin DC, Cupps RE, Johnson EW, Jr. Giant-cell tumor: a study of 195 cases. Cancer 1970;25(5):1061-1070.
pubmed doi - Tang JY, Wang CK, Su YC, Yang SF, Huang MY, Huang CJ. MRI appearance of giant cell tumor of the lateral skull base: a case report. Clin Imaging 2003;27(1):27-30.
pubmed doi - Mirra JM. Giant cell tumor (osteoclastoma). Bone Tumors. Philadelphia: Lea & Febiger, 1989:119–40.
- Harris AE, Beckner ME, Barnes L, Kassam A, Horowitz M. Giant cell tumor of the skull: a case report and review of the literature. Surg Neurol 2004;61(3):274-277.
pubmed doi - Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res 2005;435:211-218.
pubmed - Findlay JM, Chiasson D, Hudson AR, Chui M. Giant-cell tumor of the middle cranial fossa. Case report. J Neurosurg 1987;66(6):924-928.
pubmed doi - Nair SB, Abou-Elhamd KA, Hawthorne M. A retrospective analysis of high resolution computed tomography in the assessment of cochlear implant patients. Clin Otolaryngol Allied Sci 2000;25(1):55-61.
pubmed doi - Malone S, O'Sullivan B, Catton C, Bell R, Fornasier V, Davis A. Long-term follow-up of efficacy and safety of megavoltage radiotherapy in high-risk giant cell tumors of bone. Int J Radiat Oncol Biol Phys 1995;33(3):689-694.
pubmed doi - Hug EB, Muenter MW, Adams JA, de Vries A, Rosenberg AE, Munzenrider JE. 3-D-conformal radiation therapy for pediatric giant cell tumors of the skull base. Strahlenther Onkol 2002;178(5):239-244.
pubmed doi - Pai SB, Lalitha RM, Prasad K, Rao SG, Harish K. Giant cell tumor of the temporal bone—a case report. BMC Ear Nose Throat Disord 2005;5:8.
pubmed - Yamamoto M, Fukushima T, Sakamoto S, Tomonaga M. Giant cell tumor of the sphenoid bone: long-term follow-up of two cases after chemotherapy. Surg Neurol 1998;49(5):547-552.
pubmed doi - Qureshi SS, Puri A, Agarwal M, Desai S, Jambhekar N. Recurrent giant cell tumor of bone with simultaneous regional lymph node and pulmonary metastases. Skeletal Radiol 2005;34(4):225-228.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


