| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 5, Number 4, April 2014, pages 197-201
Parsonage-Turner Syndrome Initially Suspected of Being Orthopedic Diseases in a Primary Care Setting: A Case Report
Hiroki Yabea, b, Machiko Kimuraa, c, Akira Ishiia, Tamami Watanabea, Yoshio Sakiyamad, Mieko Otsukad, Hitoshi Sugawaraa, e
aDivision of General Medicine, First Department of Comprehensive Medicine, Saitama Medical Center, Jichi Medical University, Saitama City, Japan
bDivision of Rheumatology, First Department of Comprehensive Medicine, Saitama Medical Center, Jichi Medical University, Saitama City, Japan
cDepartment of Ophthalmology, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan
dDivision of Neurology, First Department of Comprehensive Medicine, Saitama Medical Center, Jichi Medical University, Saitama City, Japan
eCorresponding author: Hitoshi Sugawara, Division of General Medicine, First Department of Comprehensive Medicine, Saitama Medical Center, Jichi Medical University, 1-847 Amanuma-cho, Omiya-ku, Saitama City, Saitama 330-8503, Japan
Manuscript accepted for publication February 11, 2014
Short title: A Case Report of Parsonage-Turner Syndrome
doi: https://doi.org/10.14740/jmc1696w
| Abstract | ▴Top |
Parsonage-Turner syndrome (PTS), also known as neuralgic amyotrophy or brachial neuritis, consists of a complex set of symptoms including sudden onset shoulder pain, usually unilaterally, followed by progressive neurologic deficits such as motor weakness, dysesthesia and numbness. We report a case of a 45-year-old Japanese man with PTS, who was initially suspected of having orthopedic diseases, such as cervical spondylosis and suprascapular nerve entrapment syndrome. The patient presented with acute-onset severe pain in his right arm and neck, followed by muscle weakness. We diagnosed PTS by taking a detailed medical history, ruling out other differential diagnoses, and by considering the characteristic clinical symptoms and the denervation pattern identified by needle electromyography. High-dose corticosteroid therapy prednisolone (PSL) relieved the initial sharp pain and oral amitriptyline successfully relieved the residual nighttime pain. Doses of PSL and amitriptyline were tapered, pain was alleviated, and the patient returned to full-time work after discharge in one-and-a-half years. For prompt diagnosis, physicians should consider the possibility of PTS in patients with acute onset of severe arm pain or subsequent muscle weakness and muscle atrophy in the arm or shoulder girdle.
Keywords: Parsonage-Turner syndrome; Neuralgic amyotrophy; Brachial neuritis; Shoulder pain; Corticosteroid; Amitriptyline
| Introduction | ▴Top |
Parsonage-Turner syndrome (PTS) is rare peripheral neuropathy characterized by the acute onset of severe pain, muscle weakness and sensory disturbance. PTS is diagnosed clinically. Pain is often described as extreme, sharp or hyperpathia-like thalamic pain [1]. Suprascapular and axillary nerves and their corresponding muscles are affected most frequently [2]. PTS was first defined by Parsonage and Turner in 1948 [3]. PTS occurrence is reported to be two to three cases out of 100,000 [1]. However, because PTS is frequently misdiagnosed and is mostly under-recognized, its true incidence is estimated to be up to 10 times higher [1].
In this report, we describe a case of PTS that several other clinics had failed to properly diagnose and treat. A delayed diagnosis of PTS results in continuous severe pain and a reduction in activities of daily living (ADL) for patients [1]. Although PTS is a rare disease, a prompt diagnosis and effective therapeutic interventions are especially important. In this report, we present our case and review previous reports of PTS.
| Case Report | ▴Top |
A 45-year-old Japanese man experienced severe pain with acute onset in his right arm and neck 40 days prior to admission. The man had no known trigger for the pain, common cold or vaccinations prior to pain onset. His pain was very severe and did not resolve spontaneously; therefore, he went to an orthopedic clinic for check-up. A plain radiography of his neck revealed no abnormal findings. Non-steroidal anti-inflammatory drugs failed to relieve the pain, and the patient became unable to work.
Three weeks before admission, another orthopedic clinic suspected cervical spondylosis; however, magnetic resonance imaging (MRI) revealed no abnormal findings. One week later, his laboratory data indicated elevated white blood cell (WBC) count and C-reactive protein (CRP). The previous doctor suspected suprascapular nerve entrapment syndrome; therefore, a suprascapular nerve block was applied and the patient’s pain subsided temporarily. However, the patient subsequently had a relapse, experienced continuous severe pain, and eventually became bedridden. Thus, he was admitted to the emergency department of our medical center for further investigation.
Past medical history of the patient included a gastric ulcer at age 35 and herpes zoster on his left chest at age 37. He had no significant medical history. He smoked 10 cigarettes per day; drank 350 mL of beer per week; and took pregabalin, neurotrophin, eperisone hydrochloride, tocopheryl acetate and mecobalamin. He had no allergies, and his work involved sitting at a desk. He had no previous relevant family history.
During the initial physical examination, the patient complained of sharp pain in his right shoulder, arm and neck as well as severe right axillar tenderness. He had difficulty sitting because of the pain. He described his pain as similar to having been “bitten by a fox”. His body mass index was 25.3 kg/m2; blood pressure, 138/81 mmHg; heart rate, 61 beats/min (regular); body temperature, 35.4 °C; respiratory rate, 30 breaths/min; SpO2, 96% (room air); and his respiratory sounds were normal. He had no abdominal pain or discomfort, but had tender points along his right brachial plexus. He had difficulty extending his neck and rotating it to the right. His supraspinatus (SSP) and infraspinatus (ISP) muscles looked atrophic (Fig. 1a). His cranial nerves were normal. Manual muscle testing (MMT) revealed muscle weakness at the right C6-Th1 level. He had decreased sensation of pain at the right C5-Th2 level. Biceps reflex, triceps reflex and brachioradialis reflex on the right arm were decreased. There were neither pathological reflexes nor signs of Horner syndrome (Fig. 1b).
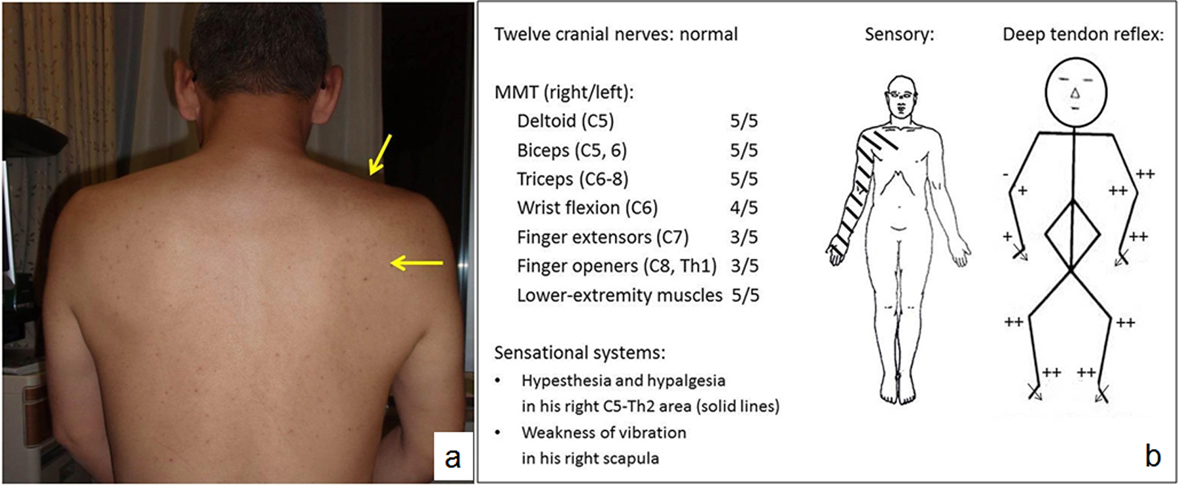 Click for large image | Figure 1. a) A picture of the patient’s back. Muscle atrophy of the right supraspinatus muscle (SSP) and infraspinatus muscle (ISP) are observed (arrow). b) The findings of neurological examinations. MMT reveals muscle weakness at the right C6-Th1 levels. Sensory tests show weak touch and pain sensations in the right C5-Th2 area. Biceps and brachioradialis reflexes are weak. Triceps reflex is negative. MMT: manual muscle testing. |
Laboratory findings were as follows: WBC, 11,560/mL; CRP, 0.19 mg/dL; aspartate transaminase, 44 IU/L; alanine transaminase, 91 IU/L; creatine kinase (CK), 49 IU/L; and erythrocyte sedimentation rate (ESR), 20 mm/h. Cerebrospinal fluid examination revealed a protein concentration of 38 mg/dL and a total cell count of 1 mononuclear cell/mL, both of which were normal. Both chest radiography and electrocardiography showed normal findings, and a nerve conduction velocity study showed no delay and no abnormal amplitude. Needle electromyography (nEMG) showed neuromuscular units (NMUs) of high amplitude and long duration in the ISP, triceps, brachioradialis and first dorsal interosseus muscles (Fig. 2). These findings indicated a peripheral nerve disorder derived from C5 through C8 nerve roots. The patient was diagnosed with PTS based on his past medical history, physical examination and electrophysiological studies.
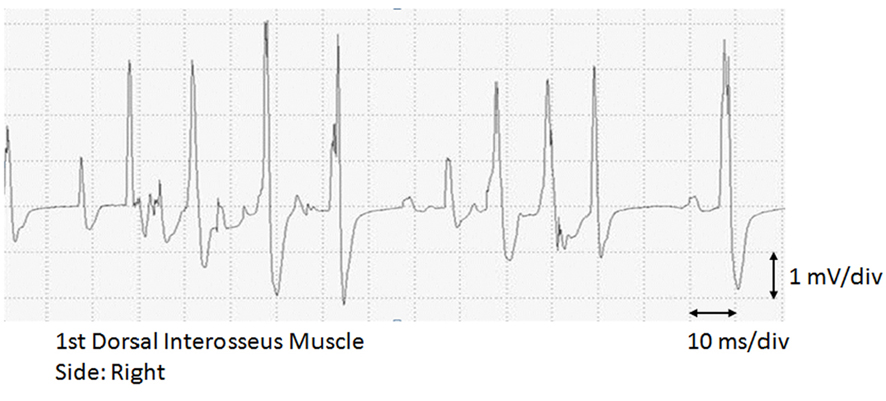 Click for large image | Figure 2. Erosseus muscle. Giant spikes of high amplitude and long duratiolectromyogram (EMG) of the first dorsal inten are shown. div: division. |
The patient’s clinical course starting from admission is shown in Figure 3. He was treated according to the suggestion described by van Alfen and van Engelen in 2006 [4]. The patient was treated with a high dose (60 mg/day) of PSL on hospital day (HD) 3. This medication decreased his daytime pain, with the numeric rating scale (NRS) pain score decreasing from 9/10 to 3/10. The ESR level was reduced from 20 mm/h to 11 mm/h at HD 12. In contrast, his severe nighttime pain persisted; therefore, he was given oral amitriptyline at 10 mg/day starting from HD 10. Amitriptyline dosage was increased to 20 mg/day by HD 12.
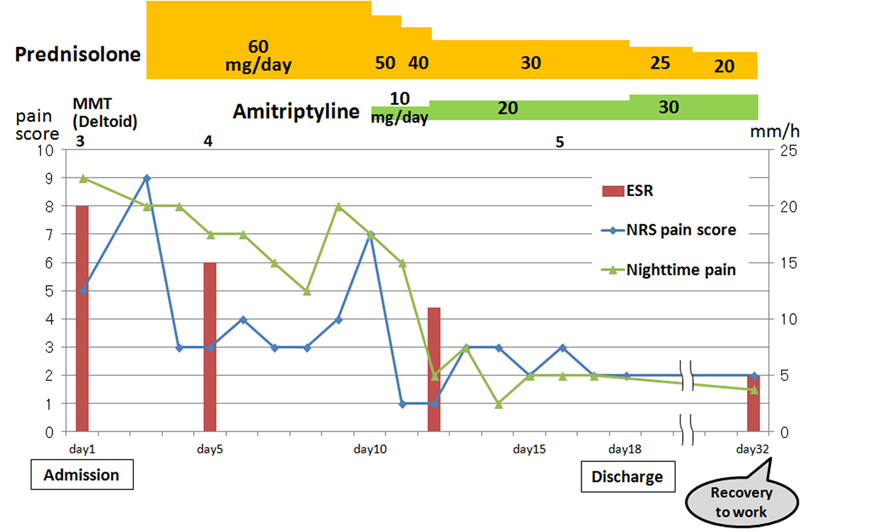 Click for large image | Figure 3. Clinical course of the patient. ESR: erythrocyte sedimentation rate; MMT: manual muscle testing; NRS: numeric rating scale; PSL: prednisolone. |
The patient’s severe nighttime pain subsided after taking amitriptyline and he was able to sleep well. Oral PSL was reduced by 10 mg/day from HD 10. When the dose was reduced to 30 mg/day, the pain score elevated slightly. Oral PSL at 30 mg/day was continued for a week. His pain gradually improved and he completely recovered from his muscle weakness on HD 16. He was discharged on HD 18.
At our outpatient clinic, oral PSL was reduced by 5 mg/week, and the patient was able to return to work 2 weeks after discharge. After 6 months, his pain decreased to 1/10-2/10 on NRS. After 8 months, follow-up nEMG showed a decrease in high and long NMUs, and after 9 months, he no longer had any sensory disturbances.
Both PSL and amitriptyline doses were tapered slowly. The patient was without pain and able to work full time after discharge in one-and-a-half years.
| Discussion | ▴Top |
PTS can have a broad range of clinical manifestations; therefore, patients frequently present to practitioners of different subspecialties, such as orthopedics, neurology or general medicine [2]. PTS is one of the causes of severe cervico-omo-brachial pain, which physicians should be aware of in the primary care setting.
A thorough medical history and complete physical examination are needed for PTS diagnosis; imaging and electromyography (EMG) can be helpful in evaluation. The highest incidence of PTS occurs in individuals between the third and seventh decades [5].
The precise cause of PTS is unknown; however, possible contributing factors have been identified. Point mutations or duplications in the SEPT9 gene on chromosome 17q25, which encodes the protein septin-9, have been implicated because these mutations can cause hereditary neuralgic amyotrophy [6]. Many potential factors associated with PTS have been reported, including infections by Epstein-Barr virus, varicella-zoster virus, cytomegalovirus, human immunodeficiency virus, Mycobacterium tuberculosis and Salmonella. Tetanus and hepatitis B immunizations have been implicated in PTS in addition to perioperative stress including lumbar punctures and herniorrhaphies, puerperium, unusual physical exercise including heavy labor, burns, medications such as abacavir and infliximab, as well as other causes [1, 2]. We could not detect any of these antecedent events in the present case.
The upper trunk of the brachial plexus is usually affected in PTS. In some patients, any part of the brachial plexus, lumbosacral plexus or any other peripheral nerves can also be affected. In an unusual case report, a patient developed dyspnea because of unilateral phrenic nerve paresis 2 years after an acute episode of PTS involving the right arm [7].
Muscle weakness develops within 1 month in 85% of cases; commonly affected muscles include the SSP, ISP, serratus anterior, biceps, deltoid and triceps [2, 8]. In 5-10% of patients, mononeuritis is observed including anterior interosseous neuritis or posterior interosseous neuritis [9]. Clinically, unilateral lesions are dominant and seen in 70% to more than 95% of patients; however, bilateral lesions are often found on EMG [1, 9].
By taking a detailed medical history, performing physical examinations, and evaluating radiographic and MR images, we were able to rule out orthopedic diseases such as cervical disk disease, cervical spondylosis, neoplastic lesions and acute calcific tendinitis. Suprascapular nerve entrapment syndrome is a representative disorder that presents muscle atrophy of the SSP and ISP. In this case, the affected nerve area was not localized to the region of the posterior axilla and extended to the brachial plexus area. Accordingly, suprascapular nerve entrapment syndrome seemed to be less likely. Mononeuropathy multiplex was unlikely because the patient did not present patchy sensory deficits. When CK levels are normal, myopathies such as muscle dystrophy and inflammatory myositis are unlikely. The diagnosis of PTS was based on the presence of severe and persistent pain, the findings of muscle atrophy of the SSP and ISP, as well as muscle weakness in the brachial plexus area and denervation patterns on nEMG.
PTS had been considered a self-limiting disorder showing good recovery without specific treatment. However, recent studies have shown that persistent pain and paresis is experienced by 50-75% of patients, with a marked effect on their ADL and working ability [1, 10].
To date, no proven effective treatment had been available for PTS. van Alfen suggested that administration of oral PSL during the first month of an attack could shorten the duration of painful symptoms and could accelerate recovery in some patients [1, 4]. The dose regimen described in that study varied between patients, and he proposed that patients take oral PSL at a daily dose of 1 mg/kg for 1 week and gradually stop the medication during the second week [1, 11]. Recently, Naito et al reported that intravenous immunoglobulin with methylprednisolone pulse therapy may be a potential treatment for PTS in which the disease is detected 1 month or more after onset [12].
In the present case, severe and persistent pain continued for a long time and it interfered with the ADL; therefore, early therapeutic interventions were needed. High-dose corticosteroid was administered one-and-a-half months after symptom onset, which was effective for daytime pain. However, nighttime pain persisted. Amitriptyline is recommended as part of the treatment of neuropathic pain [13]; therefore, it was used in this case to effectively treat nighttime pain without obvious side effects. Although the efficacy of amitriptyline in PTS has not yet been reported, it may be useful as an additional therapeutic approach for the persistent pain of PTS. In this case report, the gradual reduction of PSL was slower than that suggested by van Alfen [4] for residual pain. This might be because of the delay in the commencement of steroid therapy.
In conclusion, PTS is difficult to diagnose if physicians are not aware of it. Its underdiagnosis and misdiagnosis result in severe long-term pain, delay in starting treatment, and a decrease in the ADL. For these reasons, early diagnosis is very important. Physicians should consider the possibility of PTS when they encounter a patient with acute onset of unusual and severe arm pain or subsequent muscle weakness and muscle atrophy in the arm or shoulder girdle.
Acknowledgments
We would like to thank Nanae Kiryu (Division of Clinical Laboratory, Saitama Medical Center, Jichi Medical University) for her technical assistance.
Grant Support
We have no grant support associated with this publication.
Conflict of Interest
All authors report no conflicts of interest associated with this publication, and there has been no financial support for this paper that could have influenced its conclusions.
| References | ▴Top |
- van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat Rev Neurol. 2011;7(6):315-322.
doi pubmed - Tjoumakaris FP, Anakwenze OA, Kancherla V, Pulos N. Neuralgic amyotrophy (Parsonage-Turner syndrome). J Am Acad Orthop Surg. 2012;20(7):443-449.
doi pubmed - Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
doi - van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(Pt 2):438-450.
pubmed - Feinberg JH, Radecki J. Parsonage-turner syndrome. HSS J. 2010;6(2):199-205.
doi pubmed - van Alfen N, Hannibal MC, Chance PF, van Engelen BGM. Hereditary Neuralgic Amyotrophy. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. Gene Reviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2013. 2008 Feb 27 [updated 2012 Dec 06].
- Marvisi M, Balzarini L, Mancini C, Confortini M, Betri E. A Rare Case of Dyspnoea the Parsonage-Turner Syndrome. J Med Cases. 2012;3(3):169-171.
- Sathasivam S, Lecky B, Manohar R, Selvan A. Neuralgic amyotrophy. J Bone Joint Surg Br. 2008;90(5):550-553.
doi pubmed - Kawakita S. 3 cases of neuralgic amyotrophy with different diagnosis in initial rehabilitation. Journal of Clinical Rehabilitation. 2010;19(9):903-907.
- Geertzen JH, Groothoff JW, Nicolai JP, Rietman JS. Brachial plexus neuropathy. A long-term outcome study. J Hand Surg Br. 2000;25(5):461-464.
- van Eijk JJ, van Alfen N, Berrevoets M, van der Wilt GJ, Pillen S, van Engelen BG. Evaluation of prednisolone treatment in the acute phase of neuralgic amyotrophy: an observational study. J Neurol Neurosurg Psychiatry. 2009;80(10):1120-1124.
doi pubmed - Naito KS, Fukushima K, Suzuki S, Kuwahara M, Morita H, Kusunoki S, Ikeda S. Intravenous immunoglobulin (IVIg) with methylprednisolone pulse therapy for motor impairment of neuralgic amyotrophy: clinical observations in 10 cases. Intern Med. 2012;51(12):1493-1500.
doi pubmed - Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;12:CD008242.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


