| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 2, Number 3, June 2011, pages 132-137
Acquired Factor V Inhibitor Developing After Percutaneous Ethanol Injection Therapy for Hepatocellular Carcinoma
Yoshitaka Maedaa, c, Naoki Kojimab, Yuya Arakia, Tomomi Unoa, Tamaki Kuyamaa, Keisuke Nishigakia
aNephrology Division, Department of Internal Medicine, JA Toride Medical Center, 2-1-1 Hongo, Toride, Ibaraki 302-0022, Japan
bGastroenterology Division, Department of Internal Medicine, JA Toride Medical Center, 2-1-1 Hongo, Toride, Ibaraki 302-0022, Japan
cCorresponding author: Yoshitaka Maeda
Manuscript accepted for publication May 3, 2011
Short title: Factor V Inhibitors After PEIT
doi: https://doi.org/10.4021/jmc214w
| Abstract | ▴Top |
We report a case of an 80-year-old man who showed an abrupt-onset prolongation of the prothrombin time (PT) and activated partial thromboplastin time (APTT) one month after percutaneous ethanol injection therapy (PEIT) for hepatocellular carcinoma. The patient had chronic hepatitis (not liver cirrhosis), but the liver function had been preserved before PEIT. Supplementation of vitamin K and fresh frozen plasma could not restore the coagulation disturbance, and the following examination revealed the existence of factor V inhibitors. Because the patient was positive for hepatitis C virus and concurrent with advanced chronic renal failure, a careful follow-up without any aggressive therapy, such as administration of steroids and other immunosuppressants, was selected. The PT and APTT had been gradually normalized in the following observation period for several months, and no serious event related to bleeding tendency had occurred. This is the first report of factor V inhibitors arising after PEIT that spontaneously diminished by no additional therapy.
Keywords: Coagulopathy; Prothrombin time; Chronic kidney disease (CKD)
| Introduction | ▴Top |
The coagulopathy is one of major concerns in advanced liver diseases [1], and bleeding tendency is a common problem in patients with liver cirrhosis and hepatocellular carcinoma. But the coagulopathy is usually developed in a relatively advanced stage of liver diseases, gradually progressive and accompanied by other symptoms of liver failure, such as thrombocytopenia, esophageal varices, and encephalopathy. We encountered a case showing an abrupt prolongation in either prothrombin time (PT) or activated partial thrombin time (APTT) that was not recovered by supplementation of vitamin K and fresh frozen plasma (FFP). The following examination revealed factor V inhibitor and their relation to percutaneous ethanol injection therapy (PEIT) for hepatocellular carcinoma (HCC). Factor V inhibitor has been reported in various diseases and in conditions related to medical procedures, especially in using bovine thrombin [2-5]. However, there is no report concerning factor V inhibitor caused by PEIT that is considered to be relatively safe among invasive therapies for HCC [6, 7]. Except for self-limited complications, such as local hot sensation, pain, drunkenness and fever, the incidence of severe complications was reported to be 1.7% [6]. In the case reported here, the liver function seemed mostly preserved after PEIT. However, the case showed abrupt coagulation disturbance a month after PEIT. Since no other medical intervention had ever been carried out during that period, the procedure, PEIT might have directly caused factor V inhibitor. Thus this is the first report of factor V inhibitor emerging after PEIT in a patient with HCC.
| Case Report | ▴Top |
The case was an 80-year-old man with HCV-positive HCC that was firstly found 3 years ago. Both of two liver tumors in the S6 and S7 regions, 40 mm each in diameter, were surgically removed at another hospital. The patient firstly received blood transfusion during that surgery. Then the thrombotic arterial embolization (TAE) therapy was carried out against a rupture of the tumor in the S1 region in January 2009. In September 2010, PEIT was undertaken for the tumor in the S8 region with 3.5 ml of 90% ethanol and 0.2% lidocaine chloride.Figure 1 shows the abdominal CT of the case after PEIT. Then, the plasma prothrombin time (PT) was abruptly extended from 1.23 to 5.06 in the international normalized ratio (INR) one month (26 days) after PEIT (Fig. 2), while the patient did not show any symptoms related to bleeding tendency. As Table 1 shows, hypoalbuminemia, azotemia, mild liver injury, anemia, mild thrombocytopenia and elevated levels of AFP, along with coagulation disturbance were observed. These parameters except for the coagulation disturbance had stayed in the similar levels before and after PEIT. Drug prescription patterns had not been changed over the period of PEIT. The patient had received blood transfusion during the admission period for the resection surgery of the tumors in the S6 and S7 region 3 years ago, but then any other biological products, such as albumin or a fibrin sealant had never been administered. As Figure 2 shows, the PT could not have been normalized by repeated administration of vitamin K (VK) and infusion of fresh frozen plasma (FFP). In the following examinations, the activity of the factor V was less than the detecting limit, and the activity of factor V inhibitor was identified using the Bethesda method as 30 Bethesda unit (BU)/ml (Table 2). As Figure 3 shows, the cross-mixing test with normal plasma showed the pattern of existence of coagulation inhibitors, rather than deficiencies of coagulation factors. The patient was high-aged with renal failure, and did not show any symptoms suggesting bleeding tendency, liver failure or progressive anemia, except for transient mild hematochezia, not accompanied by a decrease in hemoglobin (Hb) levels. Therefore, we carefully observed the patient without any additional therapy other than the administration of a long-acting erythropoietin analogue, darbepoetin (Fig. 2). The PT of the patient gradually shortened, and PT-INR turned out less than 2.0, along with diminished activity of factor V inhibitor under the detection limit, five months (157 days) after PEIT (Fig. 2).
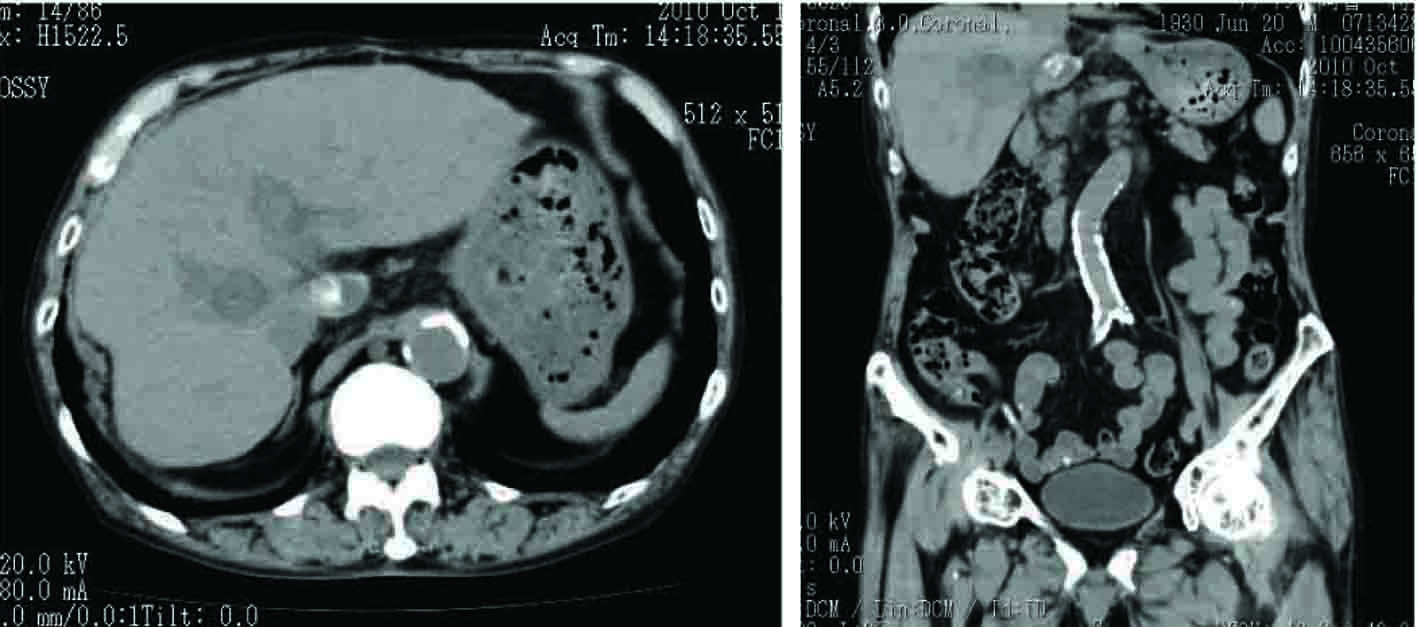 Click for large image | Figure 1. Plain abdominal CT image of the patient after PEIT. A low density SOL in deep S8 region where ethanol was injected, along with another SOL in S1 with residual ethiodized oil (Lipiodol®) was observed. |
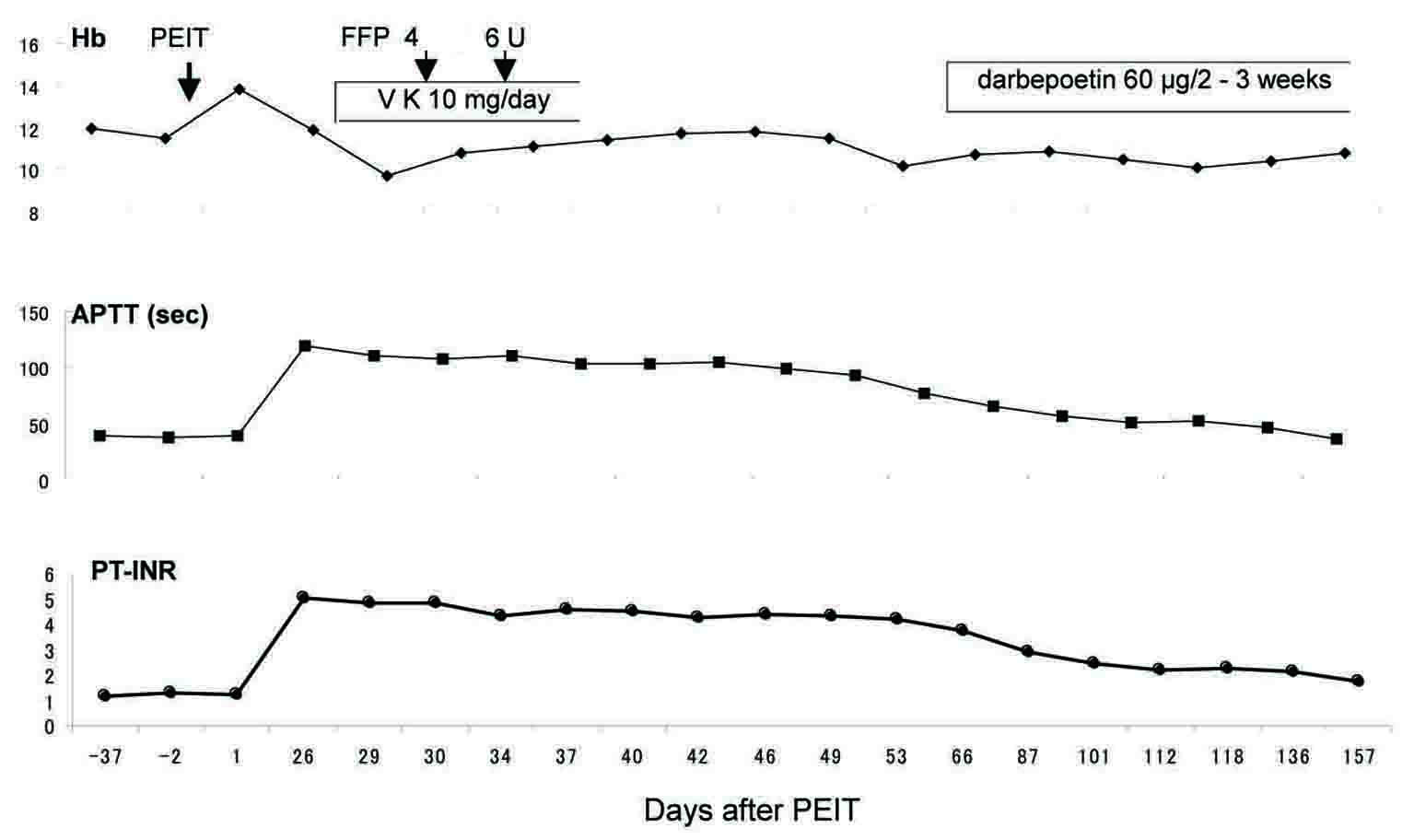 Click for large image | Figure 2. Clinical course. PT-INR was suddenly elevated 26 days after PEIT. Blood Hb levels decreased after PEIT, then stayed in 10 - 12 g/dL by additional administration of darbepoetin, although transient hematochezia had been complained on the 112th day after PEIT. The activity of factor V inhibitors was not identified (0 BU/mL) on the 157th day after PEIT. |
 Click to view | Table 1. Laboratory Findings of the Case |
 Click to view | Table 2. Laboratory Findings Related to Coagulation |
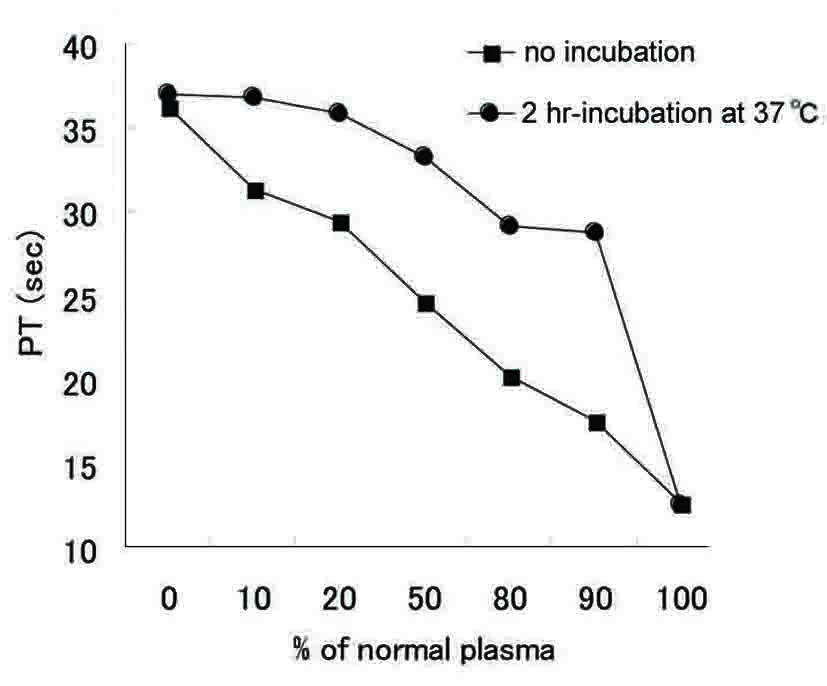 Click for large image | Figure 3. Cross-mixing test. Each PT was measured by adding the indicated amount of normal plasma to the sample obtained from the case with and without incubation. |
| Discussion | ▴Top |
We here reported the case showing the sudden-onset coagulpathy after PEIT. The other liver function had been preserved, whereas the supplementation of vitamin K and FFP failed in recovery of the coagulation function. The case showed a decline in activities of multiple coagulation factors (Table 3). The mixing test with the normal plasma suggested the existence of coagulation inhibitors, rather than a deficiency of coagulation factors, since the incubation with the normal plasma delayed the recovery of coagulation time (Fig. 3). Then the activity of factor V inhibitor was identified. The decreased activities of other coagulation factors were consistent with the previous report that suggested the possibility of interference of inhibitors to the activity assays of other coagulation factors when the titers of inhibitor stayed at higher-levels [8]. These assays measure PT or APTT of samples with the plasma that is deficient in a target coagulation factor, thus a decrease in the activity of the factor V caused by inhibitors may still affect those results. Meanwhile, the factor XIII activity using a direct photometric determination of the factor XIII [9] stayed in the normal range (Table 3). In the case reported here, although both of APTT and PT had been prolonged, the PT abnormality had been rather predominant over the entire clinical course. Therefore, factor VIII inhibitor and other coagulation abnormalities, not examined in detail in this case, were not supposed to be co-existed.
 Click to view | Table 3. Activities of the Coagulation Factors |
As summarized in Figure 4, factor V inhibitor may arise from immunological sensitization to bovine serum albumin, fibrin sealants, transfused plasma, or drugs [2]. Otherwise, impaired immunological responses may cause inhibitors in autoimmunological disorders [2, 3, 10]. But there were some cases, such as malignancies [3], in which apparent antigens could not be identified. Newly emerged antigenicities that have cross-immunogenicity with the factor V might have produced antibodies to the factor V in these cases. There is no report concerning factor V inhibitors arising from PEIT. Damaged protein exposed to ethanol consequently might have obtained a cross-antigenicity with the factor V in the case reported here, because ethanol could certainly destroy protein structures and change their antigenicities as in envelope proteins of hepatitis B virus [11]. Besides the factor V, inhibitors against the factor VIII have also been reported. The factor V and VIII have similar structures and molecular weight around 330 kilo dalton (kDa). Moreover, both proteins are spliced and inactivated by protein C bound to protein S. Hence both proteins might have some similarities in their structures that can develop cross-antigenicities with the factor V.
 Click for large image | Figure 4. Possible pathogenic mechanism of factor V inhibitors. In addition to the possibilities reported previously [2-5, 12], ethanol in this report was described as an intrinsic causative agent. |
The actual incidence of factor V inhibitors is anticipated to be higher than that of reported [12]. It will be the same or higher in patients with liver diseases, because the coagulopathy in advanced liver disease may mask a change in coagulation function [1]. In this case, factor V inhibitor had not been examined before PEIT, thus HCV infection or HCC [3] might be predisposed to developing inhibitors, although abrupt elevation of PT in the clinical course suggested a close relationship between factor V inhibitor and PEIT, rather than either HCV infection or HCC. The complete eradication of the activity of factor V inhibitor five months after PEIT, along with normalization of coagulation time also suggested a closer relationship between the emergence of inhibitor and PEIT.
Except for supplementation with fresh frozen plasma and vitamin K, no other treatments for eradicating factor V inhibitor were undertaken, because the patient had never shown any symptoms suggesting bleeding tendency and progressive anemia (Fig. 2). According to the previous reports [2, 5], severity in bleeding tendency and concurrent clinical problems varied in each case, and neither levels of PT nor titers of inhibitors were directly related to the severity and the prognosis of patients with factor V inhibitors. Moreover, some patients spontaneously recovered, and their prognoses were not different from the patients receiving therapies including steroids. These observations make it difficult to establish the treatment regimen for factor V inhibitor. Consequently, we carefully observed the patient without any additional therapies, and the patient was fully recovered. We need to accumulate cases and their information to establish a useful regimen for the management of factor V inhibitors.
In conclusion, this is the first case report of factor V inhibitor arising from PEIT for HCC, and the patient was spontaneously recovered without any treatments except for the supplementation of vitamin K and the transfusion of FFP only in the early phase of the clinical course. Although mild coagulation disturbances are frequent complications of LC and HCC, a sudden-onset coagulopathy as reported here may be derived from factor V inhibitors, especially after PEIT. Because a spontaneous recovery is anticipated in some cases as reported here, careful observations without aggressive therapies will be a choice in case of poor general condition, or with multiple concurrent problems, unless serious symptoms arise from bleeding tendency.
Acknowledgments
We sincerely appreciate the laboratory technician, Fusao Kasahara for his excellent assistance in the analysis of coagulopathy in this reported case. We also appreciate the hematologist, Takayoshi Itoh for his fruitful suggestions.
Conflict of Interest
Nothing to be declared.
| References | ▴Top |
- Senzolo M, Burra P, Cholongitas E, Burroughs AK. New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol 2006;12(48):7725-7736.
pubmed - Knobl P, Lechner K. Acquired factor V inhibitors. Baillieres Clin Haematol 1998;11(2):305-318.
pubmed doi - Endo H, Kawauchi K, Tomimatsu M, Iga D, Ogasawara T, Yasuyama M, Saito T, et al. Acquired factor V inhibitor responsive to corticosteroids in a patient with double cancers. Intern Med 2007;46(9):621-625.
pubmed doi - Nozu T, Mita H, Okumura T. Acquired factor V inhibitor in a patient with a mechanical aortic valve under warfarin therapy. Intern Med 2010;49(20):2229-2233.
pubmed doi - Ang AL, Kuperan P, Ng CH, Ng HJ. Acquired factor V inhibitor. A problem-based systematic review. Thromb Haemost 2009;101(5):852-859.
pubmed - Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 1997;12(9-10):S319-328.
pubmed doi - Lin SM, Lin DY. Percutaneous local ablation therapy in small hepatocellular carcinoma. Chang Gung Med J 2003;26(5):308-314.
pubmed - Shimizu I, Ichikawa N, Yotsumoto M, Sumi M, Ueno M, Kobayashi H. [High-titer idiopathic acquired factor V inhibitor patient showing decreased activities of multiple coagulation factors]. Rinsho Ketsueki 2008;49(6):413-416.
pubmed - Fickenscher K, Aab A, Stuber W. A photometric assay for blood coagulation factor XIII. Thromb Haemost 1991;65(5):535-540.
pubmed - Favaloro EJ, Posen J, Ramakrishna R, Soltani S, McRae S, Just S, Aboud M, et al. Factor V inhibitors: rare or not so uncommon? A multi-laboratory investigation. Blood Coagul Fibrinolysis 2004;15(8):637-647.
pubmed doi - Ito K, Kajiura T, Abe K. Effect of ethanol on antigenicity of hepatitis B virus envelope proteins. Jpn J Infect Dis 2002;55(4):117-121.
pubmed - Godart B, Boinot C, Remblier C, Hajjar A, Beauchant M. Acquired factor V inhibitor associated with valproic acid use in a cirrhotic patient. Gut 2006;55(1):134-135.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


