| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 6, Number 10, October 2015, pages 463-467
Cerebrovascular Ischemic Events in Patients With Advanced Renal Cell Carcinoma Treated With Anti-Angiogenic Tyrosine Kinase Inhibitors: A Report on Two Cases With Different Outcomes
Ankit Raoa, b, Emilio Porfiria
aThe Cancer Centre, Queen Elizabeth Hospital, Edgbaston, Birmingham B15 2TH, UK
bCorresponding Author: Ankit Rao, The Cancer Centre, Queen Elizabeth Hospital, Edgbaston, Birmingham B15 2TH, UK
Manuscript accepted for publication September 08, 2015
Short title: Cerebrovascular Ischemic Events
doi: http://dx.doi.org/10.14740/jmc2289w
| Abstract | ▴Top |
Small-molecule tyrosine kinase inhibitors (TKIs), targeting tumor angiogenesis, have revolutionized the treatment of advanced renal cell carcinoma (RCC) over the last decade. Their rationale is that most clear cell RCCs have alterations in the Von Hippel-Lindau (VHL) gene pathway that leads to over-expression of pro-angiogenic factors such as vascular endothelial growth factor and platelet-derived growth factor that drive tumor growth and dissemination. The toxicity profile of these therapies, whilst generally mild and manageable, is quite distinct from that of conventional cytotoxic chemotherapy and immunotherapy. Arterial thrombotic events have been reported with pazopanib and axitinib (1-2% incidence) and patients with recent vascular events have been excluded from phase III trials of these drugs in RCC. We report two cases of cerebral infarction likely related to these treatments in female patients without any history of macrovascular disease or any conventional risk factors such as hyperlipidemia, hypertension or diabetes mellitus. Treatment-related arterial thromboembolism may develop rapidly and unpredictably in these patients and consideration should be given to aggressively monitoring and modifying any pre-existing vascular risk factors. Older patients with risk factors for vascular disease or with a prior history of such events must have an informed discussion regarding the risks and benefits of treatment. It remains to be seen whether prophylactic anti-platelet therapies such as aspirin, dipyridamole or clopidogrel might reduce the risk of stroke in these patients with an acceptable risk of bleeding.
Keywords: Stroke; Pazopanib; Axitinib
| Introduction | ▴Top |
It is over 20 years since the discovery of frequent somatic mutations in the Von Hippel-Lindau (VHL) gene in sporadic clear cell renal cell carcinomas (RCCs) [1]. This finding led to elucidation of the pathophysiology of clear cell RCCs in terms of defective VHL gene product function leading to reduced poly-ubiquitination of hypoxia-inducible factor 1α (HIF1α), accumulation of HIF1α and increased transcription of numerous HIF1α target genes including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and erythropoietin [2].
Over the past decade, the therapeutic focus for advanced RCC has shifted from immunotherapy (using cytokines) to small-molecule tyrosine kinase inhibitor (TKI) therapies directed against over-expression of pro-angiogenic factors such as VEGF or PDGF. Several multi-targeted, predominantly anti-angiogenic small-molecule TKIs have shown robust evidence of efficacy in clear cell RCC including sunitinib [3], sorafenib [4], pazopanib [5] and axitinib [6]. A VEGF-targeted monoclonal antibody, bevacizumab, has also shown evidence of survival benefit in combination with alpha-interferon [7].
In the pivotal phase III trials of the above-mentioned TKIs, rates of treatment discontinuation due to treatment-related adverse events were 8% with sunitinib, 19% in cytokine-pre-treated and 12% in treatment-naive patients with pazopanib, 4% with sorafenib and 4% with axitinib. The most common adverse events are diarrhea, abnormalities of liver function tests (particularly with pazopanib), fatigue, hypothyroidism, hypertension, reduced appetite, nausea and poor wound healing. Returning to vascular events, myocardial dysfunction, hemorrhage, and thrombosis (arterial and venous) also occur. Most toxicities are mild and manageable with simple supportive measures or dose reduction.
An understanding of the normal physiological function of VEGF provides some degree of explanation of some of the serious “on-target” adverse events of anti-angiogenic TKIs and bevacizumab. Regulation of endothelial cell integrity, migration, proliferation and apoptosis is dependent on VEGF. Inhibition of VEGF function by pharmacological manipulations may lead to reduced nitric oxide (NO) and prostacyclin (PGI2) production which favors platelet aggregation, vasoconstriction and thrombosis [8]. Interference with endothelial cell function leads to exposure of pro-thrombotic phospholipids and underlying stroma [9].
The risk of arterial thromboembolism is particularly associated with anti-angiogenic TKIs as evidenced by the very low rates of vascular toxicity associated with the mutant BRAF inhibitor vemurafenib in melanoma [10] or erlotinib [11] in epidermal growth factor receptor mutant non-small cell lung cancer.
Here we present two cases of cerebral ischemia occurring in patients without multiple risk factors who were taking pazopanib and axitinib for metastatic RCC with differing outcomes.
| Case Reports | ▴Top |
Case 1
A 58-year-old Caucasian female presented with lethargy, exhaustion and macroscopic hematuria followed by the development of nausea, leg edema and abdominal distension. Her past medical history was remarkable only for prior left shoulder surgery and bilateral oophorectomy for endometriosis. She was a non-smoker with no family history of malignancy. CT scan demonstrated a 10 × 9 cm right renal mass with invasion of the inferior vena cava (IVC) to the level of the hepatic confluence as well as bulky retroperitoneal lymphadenopathy and a 1 cm left upper lobe lung nodule consistent with metastasis. The tumor infiltrated the surrounding musculature. CT-guided biopsy of the renal mass only showed fibro-collagenous tissue and some atypical epithelial cells that were not diagnostic of malignancy. The patient declined repeat biopsy and based on clinical and radiological features, she commenced targeted therapy with pazopanib at 600 mg daily. WHO performance status was one. Fourteen days after starting treatment, she presented with a 3-day history of severe generalized headaches and vomiting. On neurological examination, she was withdrawn, virtually aphasic with right arm and leg weakness in a pyramidal pattern. An unenhanced CT head scan demonstrated a small area of low attenuation change in the left temporal lobe. Pazopanib was withheld. Cerebrospinal fluid (CSF) analysis was performed and showed clear, colourless CSF. There was no evidence of xanthrochromia, and CSF protein and glucose were within normal limits. The CSF was acellular, PCR for viral infections was negative and the opening pressure was normal. Diffusion-weighted MRI of the brain showed a shower of acute “embolic” infarcts bilaterally with a greater degree of involvement of the frontal and parietal lobes than the occipital lobes (Fig. 1). The highest blood pressure recorded during admission was 138/81 mm Hg. Electroencephalogram showed an excess of slow activity consistent with mild diffuse cerebral dysfunction. Secondary prophylaxis against further infarction was initiated with clopidogrel and simvastatin. A search for cardiac sources of embolism was made but transthoracic echocardiography was unremarkable except for mild diastolic dysfunction (impaired relaxation) and a visible tumor mass in the IVC. Twenty-four-hour ambulatory electrocardiogram (ECG) recording showed normal sinus rhythm with infrequent multi-focal ventricular ectopic beats and single supraventricular ectopic beats. Doppler ultrasonography of the carotid arteries was unremarkable. Approximately 6 weeks after the cerebrovascular event, following a gradual improvement in neurological deficits, she started further targeted therapy with the mTOR inhibitor everolimus. Her tolerance of everolimus was poor with symptomatic anemia, stomatitis and oral ulceration, nausea and reduced appetite. A switch in treatment to the VEGFR1, 2, 3 antagonist axitinib was briefly considered but ultimately she died from progressive disease 3 months after starting everolimus.
 Click for large image | Figure 1. Diffusion-weighted MRI brain (ADC) showing multiple small areas of acute infarction. |
Case 2
A 67-year-old Caucasian female presented with cough and exertional breathlessness on a background of frequent infective exacerbations of bronchiectasis. She was an ex-smoker with a 20 pack-year history and did not drink alcohol. CT scan demonstrated a large heterogeneously enhancing right renal mass, small bilateral pulmonary nodules consistent with metastases and a 3.3 cm right hilar mass with partial collapse of the right middle lobe. There were no hepatic or skeletal metastases. She underwent palliative nephrectomy revealing a Fuhrman grade 4 pT3a clear cell RCC with sarcomatoid differentiation and necrosis. She commenced treatment with sunitinib (50 mg daily, 4 weeks on and 2 weeks off) but the dose was reduced to 37.5 mg daily due to stomatitis and oral ulceration. Treatment was complicated by hypertension (up to 170/100 mm Hg) and ramipril 5 mg daily was instituted with good effect. After 12 months of treatment, imaging demonstrated a partial response (57% reduction in disease volume). This response was ongoing for a further 9 months, at which point she developed progressive disease with enlarging aorto-caval and right hilar lymphadenopathy and new parenchymal lung metastases. Treatment was switched from sunitinib to axitinib. She tolerated axitinib well although subclinical hypothyroidism (thyroid stimulating hormone 18.55 mU/L) developed and was treated with levothyroxine replacement (25 μg daily). Treatment was interrupted after 9 weeks due to grade 3 transaminase elevation (alanine aminotransferase 282 U/mL) and she was treated with doxycycline for a suspected infective exacerbation of bronchiectasis. Three days after stopping axitinib, she presented acutely unwell with a 12-h history of constant frontal headache, visual disturbance, nausea, left facial droop, dysarthria and left arm and leg weakness. Capillary blood glucose was 5.7 mM and blood pressure was 145/87 mm Hg. On examination, there was pyramidal weakness affecting the left upper and lower limb (MRC grade 4/5), a mild facial nerve weakness, and left homonymous hemianopia. Cardiovascular and gastrointestinal examinations were unremarkable, and respiratory examination revealed expiratory wheeze and reduced air entry at the left lung base. Laboratory investigations showed platelets of 141 × 109, white blood cell count 15.7 × 109/L, absolute neutrophil count 12.2 × 109/L, alanine aminotransferase 197 U/mL, alkaline phosphatase 455 IU/L and albumen 32 g/L. Contrast-enhanced CT head showed small hypodense areas in the left cerebellum and left occipital lobe. A clinical diagnosis of acute ischemic stroke was made and she was treated with 300 mg aspirin daily. Twenty-four hours later, she became drowsy, dysarthric and inattentive and neurological examination revealed complete paralysis of the left face and upper and lower limbs, a Glasgow coma score of 12/15 (eyes 4, motor 6, verbal 2), sluggish right pupillary reaction to light, eye deviation to the right as well as 4/5 muscle power in the right upper limb and 3/5 in the right lower limb. Repeat CT head revealed no new findings, but MRI of the brain demonstrated a very large right middle cerebral artery (MCA) acute infarction (Fig. 2) with multiple small acute infarcts throughout the brain (right and left frontal and occipital lobes, left thalamus, right and left cerebellum (Fig. 3)). Digital subtraction cerebral angiogram showed absent contrast opacification of the right MCA (Fig. 4). At this point, enteral nutrition was started via naso-gastric tube. Her condition continued to deteriorate with the development of hypoxemia (SaO2 91% on FiO2 21%) and patchy consolidation throughout the right lung on chest radiograph. Despite hydration, nutrition and antibiotics, there was no clinical improvement and she died 6 days after hospital admission. The cause of death was listed as aspiration pneumonia secondary to extensive ischemic stroke.
 Click for large image | Figure 2. Diffusion-weighted MRI brain showing large right middle cerebral artery territory infarction. |
 Click for large image | Figure 3. Diffusion-weighted MRI brain showing multiple small areas of acute infarction. |
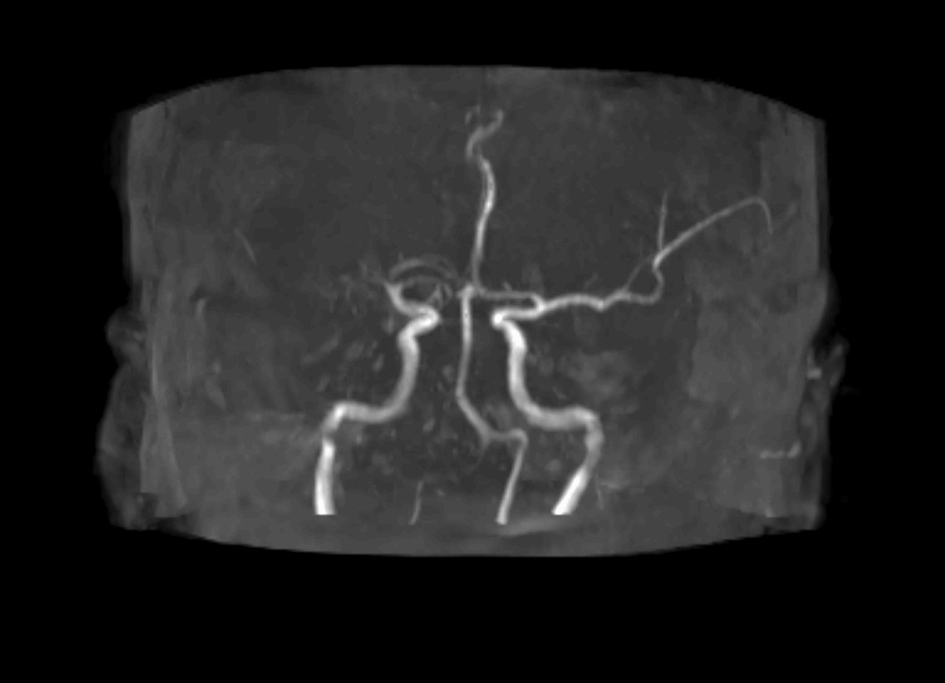 Click for large image | Figure 4. MR angiography (DSA) showing absent contrast opacification of right middle cerebral artery. |
| Discussion | ▴Top |
There is only one prior case report of an ischemic stroke in a patient with metastatic RCC on anti-angiogenic TKI therapy [12]. This was an elderly male, previously treated with nephrectomy, alpha-interferon and 5-FU, who developed an acute ischemic stroke affecting the right parietal lobe after 1 year of treatment with sunitinib with near-complete resolution of neurological deficits (left homonymous hemianopia and left hemiparesis) within 48 h. Notably, this patient had established vascular risk factors including a heavy smoking history and hypertension, whereas one of our patients had no pre-existing risk factors and one had only a moderate cigarette smoking history.
A large trial-level meta-analysis of 10,255 patients receiving anti-VEGF TKIs for solid malignancies showed an absolute risk of 1.4% for a major acute arterial thromboembolic event and a relative risk of approximately three times that in patients receiving placebo, despite exclusion of patients with a major arterial event within 6 - 12 months. In patients with RCC, the relative risk was 6 [13]. A more recent analysis of 9,711 patients from 19 randomized, controlled trials found an absolute risk of arterial thromboembolic events of 1.4% in patients receiving VEGF TKIs and an odds ratio of 2.26 with only approximately 15% of events relating to cerebral ischemia [14].
In terms of treatment-related mortality in patients with solid cancers receiving anti-VEGF therapy, a pooled analysis from 10 randomized, placebo-controlled trials suggested that the relative risk of a fatal adverse event was 2.23, although haemorrhage and myocardial infarction accounted for almost two-thirds of treatment-related fatalities and death due to ischemic stroke was extremely rare (1 in 2,461) [15].
In both the cases described, we believe that the neuroimaging findings, namely small scattered areas of infarction in multiple arterial territories, are entirely consistent with direct endothelial damage at the arteriolar or capillary levels due to pharmacologic inhibition of physiologic VEGF function. In case 1, the primary kidney cancer remained in situ and there was tumor/thrombus in the IVC and therefore the differential diagnosis of paradoxical embolism entering the right side of the heart and shunting through a right-to-left communication (such as patent foramen ovale or atrial septal defect) to the cerebral circulation has to be considered. However, the fact that the patient was treated with therapeutic doses of low-molecular weight heparin and the unremarkable ECG and transthoracic echocardiogram argue against this possibility. Neither case showed features of reversible posterior leukoencephalopathy, another form of neurological toxicity associated with anti-angiogenic TKIs [16].
In both cases (particularly with sunitinib), cerebral infarction occurred soon after initiation of treatment (2 and 9 weeks) which suggests direct toxicity rather than an indirect mechanism via conventional risk factors such as hypertension or hypothyroidism-associated hyperlipidemia. Patient 1 had, however, received 18 months of sunitinib without vascular toxicity. Of some interest, headache and nausea were presenting features in both cases and these symptoms are very unusual in ischemic strokes in general.
These cases raise pertinent questions. We believe that patients with a distant history of acute arterial thrombotic events (peripheral, cerebral, and cardiac) should be carefully counselled regarding the risks and benefits of palliative treatment with anti-angiogenic TKIs and that in such patients risk factors such as hyperlipidemia and diabetes mellitus should be tightly controlled, in conjunction with the patients’ primary care physician, prior to treatment starting. In this regard, the synergistic hepatotoxicity with pazopanib and simvastatin should be considered [17]. The fact that fatal ischemic stroke can occur should encourage greater consideration of the active surveillance approach initially for asymptomatic patients with low-volume, slowly progressive metastatic disease rather than immediate treatment. It also remains uncertain whether treatment with low-dose anti-platelet agents such as aspirin, clopidogrel or dipyridamole would be an appropriate primary prevention strategy in patients treated with anti-angiogenic TKIs in view of the well-documented increased risk of hemorrhage. If the patient described in case 2 had presented for medical evaluation within 4.5 h of symptom onset, would thrombolytic treatment have been indicated and safe to administer?
There was no evidence of thrombotic micro-angiopathy in either patient, although this has been described in association with anti-angiogenic TKI treatment previously [18]. Axitinib can be associated with elevations in hemoglobin concentration and hematocrit which might pre-dispose to arterial thrombosis [19, 20], but our patient was not polycythemic. Efforts should be made to identify biomarkers of the small subset of patients who develop clinically significant vascular toxicity as some degree of vascular dysfunction appears to be near-universal in TKI-treated patients with up to 80% of patients with advanced cancer developing troponin I elevations on anti-angiogenic therapies in one study [21].
Conclusion
These two cases illustrate that cerebrovascular ischemic events can occur in association with anti-angiogenic TKI treatment in an unpredictable fashion, within a few weeks to months of commencing treatment, and in patients who have few conventional risk factors for arterial thrombotic disease nor any prior history of such events. The pattern of vascular damage suggests widespread drug-induced endothelial damage at the arteriolar and capillary levels mimicking the picture of cardiogenic embolism. We believe that patients should be thoroughly counselled prior to treatment regarding the risk (albeit very small) of potentially fatal stroke. It is important that modifiable vascular risk factors are addressed prior to treatment. Further research is required to ascertain whether antiplatelet agents might be appropriate as primary prophylaxis in TKI-treated patients and whether the benefit would outweigh the increased hemorrhagic risk [22]. As novel therapeutic strategies emerge for metastatic RCC, such as immune-checkpoint blockade [23], the potential vascular toxicity of drugs such as pazopanib and axitinib may become particularly important.
| References | ▴Top |
- Foster K, Prowse A, van den Berg A, Fleming S, Hulsbeek MM, Crossey PA, Richards FM, et al. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994;3(12):2169-2173.
doi pubmed - Kaelin WG, Jr. The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007;13(2 Pt 2):680s-684s.
doi pubmed - Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115-124.
doi pubmed - Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312-3318.
doi pubmed - Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061-1068.
doi pubmed - Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
doi - Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103-2111.
doi - Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49(3):568-581.
doi - Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788-1795.
doi pubmed - Sharma A, Shah SR, Illum H, Dowell J. Vemurafenib: targeted inhibition of mutated BRAF for treatment of advanced melanoma and its potential in other malignancies. Drugs. 2012;72(17):2207-2222.
doi pubmed - Petrelli F, Cabiddu M, Borgonovo K, Barni S. Risk of venous and arterial thromboembolic events associated with anti-EGFR agents: a meta-analysis of randomized clinical trials. Ann Oncol. 2012;23(7):1672-1679.
doi pubmed - Lonergan MT, Kellehar F, McDermott R, Collins R. Ischaemic stroke in a patient on sunitinib. BMJ Case Rep. 2010;2010.
- Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28(13):2280-2285.
doi pubmed - Qi WX, Shen Z, Tang LN, Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol. 2014;92(2):71-82.
doi pubmed - Schutz FA, Je Y, Richards CJ, Choueiri TK. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012;30(8):871-877.
doi pubmed - Foerster R, Welzel T, Debus J, Gruellich C, Jaeger D, Potthoff K. Posterior reversible leukoencephalopathy syndrome associated with pazopanib. Case Rep Oncol. 2013;6(1):204-208.
doi pubmed - Xu CF, Xue Z, Bing N, King KS, McCann LA, de Souza PL, Goodman VL, et al. Concomitant use of pazopanib and simvastatin increases the risk of transaminase elevations in patients with cancer. Ann Oncol. 2012;23(9):2470-2471.
doi pubmed - Kapiteijn E, Brand A, Kroep J, Gelderblom H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007;18(10):1745-1747.
doi pubmed - Alexandre I, Billemont B, Meric JB, Richard S, Rixe O. Axitinib induces paradoxical erythropoietin synthesis in metastatic renal cell carcinoma. J Clin Oncol. 2009;27(3):472-473; author reply 473-474.
doi pubmed - Alexandrescu DT, McClure R, Farzanmehr H, Dasanu CA. Secondary erythrocytosis produced by the tyrosine kinase inhibitors sunitinib and sorafenib. J Clin Oncol. 2008;26(24):4047-4048.
doi pubmed - Ederhy S, Massard C, Dufaitre G, Balheda R, Meuleman C, Rocca CG, Izzedine H, et al. Frequency and management of troponin I elevation in patients treated with molecular targeted therapies in phase I trials. Invest New Drugs. 2012;30(2):611-615.
doi pubmed - Sonpavde G, Bellmunt J, Schutz F, Choueiri TK. The double edged sword of bleeding and clotting from VEGF inhibition in renal cancer patients. Curr Oncol Rep. 2012;14(4):295-306.
doi pubmed - Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. 2015;33(13):1430-1437.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


