| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 6, Number 11, November 2015, pages 527-533
“Unclassical” Combination of Smell Dysfunction, Altered Abdominal Nociception and Human Hypertension Associated “Classical” Adrenal-Augmentation
Daniel S. Leon-Arizaa, b, Juan S. Leon-Arizab, c, e, Fidias E. Leon-Sarmientod
aFaculty of Health Sciences, Universidad de Santander - UDES, Bucaramanga, Colombia
bMediciencias Research Group, Unicolciencias/Universidad Nacional, Bogota, Colombia
cFaculty of Medicine, Universidad de la Sabana, Chia, Colombia
dSmell & Taste Center, Department of Head and Neck Surgery, Perelman School of Medicine, University of Pennsylvania, PA, USA
eCorresponding Author: Juan Sebastian Leon-Ariza, Carrera 20 No. 45A - 08 (606), Mediciencias Research Group, Bogota, Colombia
Manuscript accepted for publication October 06, 2015
Short title: Smell Dysfunction and Human Hypertension
doi: http://dx.doi.org/10.14740/jmc2330w
| Abstract | ▴Top |
We report a 33-year-old female patient, who arrived to the emergency ward with an abdominal pain that suddenly started 10 days before admission. Simultaneously, the patient developed sudden arterial hypertension and smell disturbances. Conventional medical treatment for pain and arterial hypertension was effortless. Laboratory tests ruled out pancreatitis. Metanephrines in her urine were also normal. A dual-phase intravenous contrast computed tomography of the abdomen showed a large mass within left adrenal gland. Adrenocortical adenoma was diagnosed. The mass was not hypervascularized but positive for synaptophysin and chromogranin A. Importantly, these proteins are heavily involved with acetylcholine metabolism. The triad of olfactory disorders, pain and arterial hypertension normalized after surgically extracting the adrenal mass. To our knowledge, this medical case is the first reported patient exhibiting immediate recovery of such unclassical triad of local and remote findings. The function and dysfunction of key nanocholinergic pathways involved with smell, blood pressure and nociception would explain the pathophysiology of this unique medical case.
Keywords: Smell; Arterial hypertension; Pain; Synaptophysin; Chromogranin A; Adrenal adenoma
| Introduction | ▴Top |
Abdominal pain is a biomedical problem facing numerous unspecific or idiopathic etiologies. Similar situations happen with human arterial hypertension. In many instances, these two medical conditions correlate [1, 2]. If these medical conditions are improperly treated, the outcome is often fatal. This complexity gets worse when unexpected and remote anomalies debut. Together, it makes more difficult to understand and bring under control not only altered nociception and disturbed blood pressure but associated unclassical chemosensory dysfunctions.
For the first time, a patient who had both local and remote adrenal mass-associated complaints that were refractory to conventional medications is reported. Importantly, a singular nanopathophysiological picture surfaces from the nanodisturbances associated to the radiologically identified mass here. In fact, the novel pathophysiological picture presented here allowed us to explain not only the sudden and pharmacologically resistant arterial hypertension, the abruptly altered abdominal nociception, and the unexpected olfactory loss that accompanied the somatic anomalies experienced by this patient but also the rather quick post-surgical recovery of the abovementioned triad of complains.
| Case Report | ▴Top |
A 33-year-old female patient was admitted to the emergency room (ER) of a university hospital located in Colombia, South America. The patient complained of abrupt abdominal pain 10 days before admission. The pain was described as excruciating cramping, that started in the epigastrium and radiated in a band-like form to the lower back and right paraspinal areas. Incidentally, the patient mentioned that she noticed loss of smell on the same day her abdominal pain started. No hematemesis was reported. The rest of her past medical history was unremarkable.
The patient arrived to the ER conscious, hydrated and afebrile. At that time her blood pressure was 220/140 mm Hg. Her heart rate was 141 beats/min. Her respiratory rate was 24 breaths/min. Diarrhea and persistent emesis of food content occurred during the day of admission to the ER. After this episode, bilateral loss of smell was confirmed by the patient. Soft depressible abdomen with tenderness in the epigastrium and bilateral fist positive percussion was noted. The remaining physical evaluation was unremarkable.
A presumptive diagnosis of pancreatitis and hypertension emergency was done. Intravenous tramadol and dipyrone did not relieve pain. Hypertension did not resolve after a number of medications administered following international guidelines (e.g., labetalol, enalapril, hydrochlorothiazide, metoprolol and amlodipine). Having at front a challenging case, a number of evaluations were done, as explained below.
Statistical analysis and ethics
T-test was used to analyze heart rate, blood pressure values, pain measures and smell scores obtained before and after surgery (see below). P value was set at 0.05. Written and informed consent from the patient was obtained before doing all of the laboratory evaluations.
Laboratory investigations
Blood count, blood electrolytes, liver and renal function tests, clotting times, thyroid hormone levels, alanine aminotransferase, serum amylase and lipase were measured. Adrenal function tests (e.g., ACTH levels, cortisol rhythm and dexamethasone suppression tests) were not considered at admission. However, due to the uncontrolled and chaotic symptoms, a 24-h urinary metanephrines test was ordered 4 days after admission to the ER.
Alanine aminotransferase was slightly elevated (55.1 U/L). Mild hypokalemia (2.5 mEq/L) was found. Metanephrines (vanillylmandelic acid: 6.3 mg; homovanillic acid: 5 µg/mg of creatinine) as well as other laboratory tests was within normal limits.
Imaging studies
M-mode two-dimensional echocardiogram was done to rule out cardiac incidentaloma. Hepatobiliary ultrasonography was performed to rule out acute pancreatitis. Since the findings obtained with the mentioned studies were inconclusive, a dual-phase intravenous contrast computed tomography of the abdomen was performed. Infusion rate was kept constant during tomography at 3 - 5 mL/s [3].
Ecography showed a slight and unspecific increase of pancreas echogenicity. No dilation of intra- or extra-hepatobiliary tract was observed. The abdominal tomography showed a regular uniform mass. It had well-demarcated margins and homogeneously low density. No calcification, necrosis or cystic degeneration was observed. The size of the mass was of 4 cm diameter. It was located within the left adrenal gland (Fig. 1). No hypervascularization was found on arterial-phase CTs. These findings were compatible with adrenocortical adenoma. No further images were needed [3].
 Click for large image | Figure 1. Arterial-phase enhanced computed tomography. Axial view (left), coronal view (right). Images show a homogenous solid mass with a diameter of about 4 cm, in left hemiabdomen (white arrow). The mass has a well-demarcated margin, and homogeneously low density. Calcification, necrosis or cystic degeneration was not observed. The mass located within the left adrenal gland is also compromising left kidney. The atrophy and loss of cortico-medullary relationship of the left kidney was noteworthy. Asymmetrical renal volume was evident; however, renal obstruction was not. |
Needle biopsy
Fine needle aspiration was made under CT. Direct smears were air dried for staining (Stat Lab, Lewisville, TX, USA), and assessed immediately by a cytopathologist. Immunostaining was performed at the time of cytologic diagnosis. Antibodies against pancytokeratin, A103, calretinin, inhibin, vimentin, synaptophysin (SNP), chromogranin A (CGA), S-100, and HMB-45 were used. The sources and the dilutions of these antibodies followed those published elsewhere [4].
No mitosis or anaplastic changes were present. Necrosis or calcifications were not seen. Lipid-laden cells arranged in nets were reported. Cortical adenoma was diagnosed. The mass showed widespread SNP and CGA reactivity [4]. P450c21 (steroid 21-hydroxylase) [5] was not tested due to lack of technical facilities for testing this antibody [6]. A wider discussion on the macroscopic and cytological features on background content, cellularity, architecture, cell shape, cytoplasmic and nuclear features as well as immunostaining will be done elsewhere.
Autonomic function
The heart rate in beats/min and the blood pressure in mm Hg were measured at rest, while lying down for 3 min [7], during three consecutive days each. For our analysis, we include the data obtained at 8:00 am.
Heart rate (140.3 ± 2.08 beats/min), systolic blood pressure (219.6 ± 2.51 mm Hg) and diastolic blood pressure (123.3 ± 8.96 mm Hg) were abnormally increased before surgery. Both of these measurements returned to normal values immediately after resection of the mass (Fig. 2) (heart rate: 83.6 ± 3.05 beats/min; systolic blood pressure: 123.3 ± 8.96 mm Hg; diastolic blood pressure: 85.6 ± 3.5 mm Hg) remaining similar during the rest of hospitalization. The changes in values obtained after surgery were statistically different to the values obtained before mass removal (systolic blood pressure: T-test (t) = 25.0595; degree of freedom (df) = 2; P = 0.0016; diastolic blood pressure: t = 32.6000; df = 2; P = 0.0009; hear rate: t = 25.9248; df = 2; P = 0.0015).
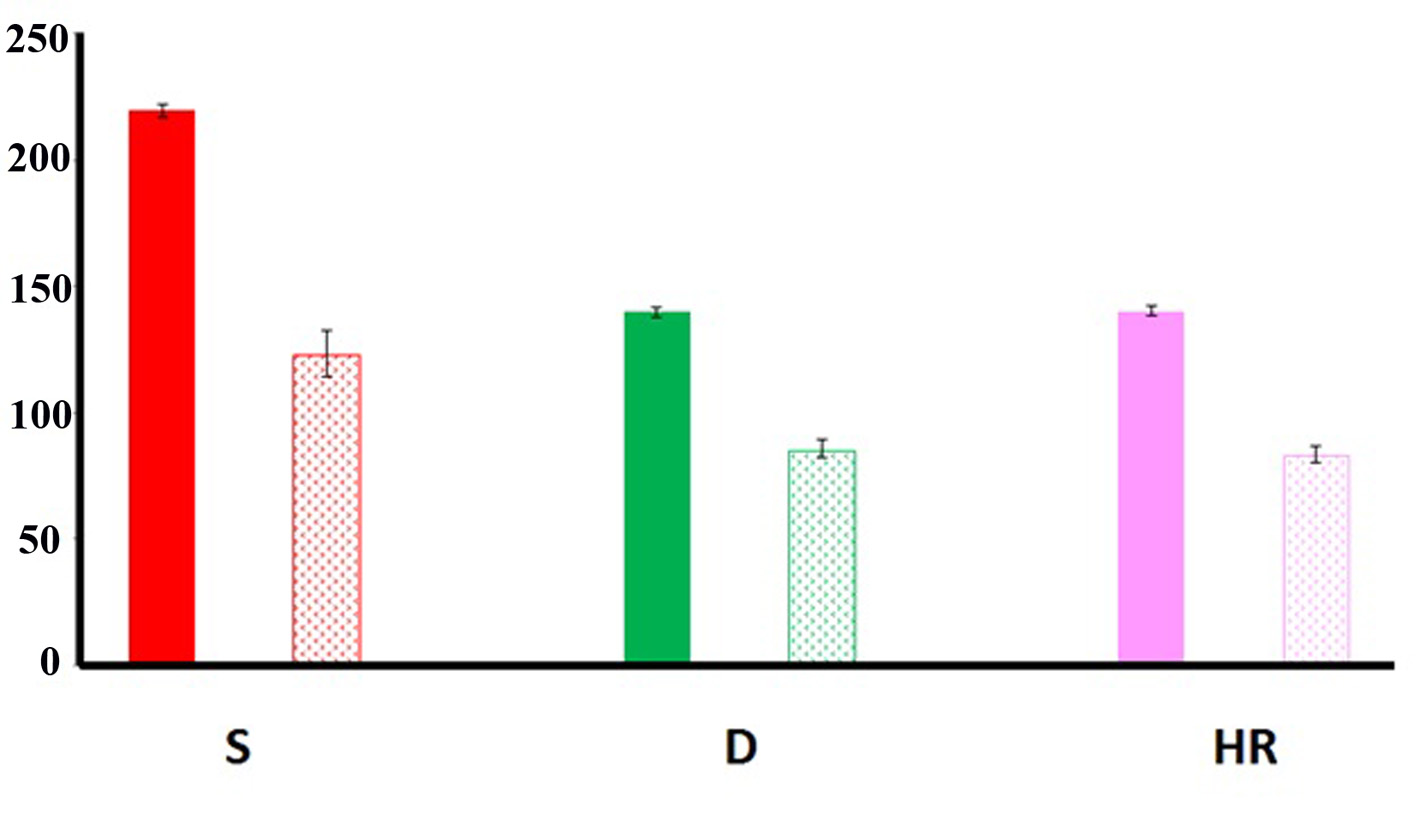 Click for large image | Figure 2. Autonomic function. Systolic (S), diastolic (D) and heart rate (HR) values obtained before (filled bars) and after (dotted bars) surgical removal of the adrenal adenoma. Note that the values obtained after surgery significantly improved (see text for details). Bars: standard deviation. |
Nociceptive evaluation
Self-report of pain was measured using a 100-mm visual analogue scale (VAS). Verbal anchors at either end were used (0 mm = no pain; 100 mm = “as bad as can possibly be”). The administration of this tool is easy, and it is considered a sensitive and accurate measure of pain intensity. Advanced reading levels are not required by the subject to quantify the pain [5]. Pain ratings were made at the same time when blood pressure and heart rate measurements were made.
The abdominal pain declared by the patient before the surgical procedure (VAS: 9.6 ± 0.47) went away post-surgical adrenal mass excision (Fig. 3). The post-surgical values obtained with the VAS were much lower (1.33 ± 0.47), and different statistically compared to those obtained before surgery (t = 25.0000; df = 2; P = 0.0016).
 Click for large image | Figure 3. Nociceptive evaluation. The values obtained by applying the visual analog scale (VAS) significantly decreased after mass removal (AS) compared to the values obtained before surgical intervention (BS) (see text for details). Bars: standard deviation. |
Olfactory quantification
A validated version of the University of Pennsylvania smell identification test (UPSIT) was administered. The UPSIT is comprised of four booklets, each containing 10 microencapsulated odorants with one odorant per page [8]. A multiple-choice question with four response alternatives per item is located above each odorized strip and the subject must choose the alternative that best represents the perceived smell. The UPSIT was administered before and after surgery (see below), two times each. The picture identification test (PIT), a test analogous to the UPSIT except that there are pictures rather than odors serving as test stimuli, was also administered after the application of the UPSIT [8]. This test was applied along with the UPSIT to be certain that the obtained scores were not confounded by cognitive problems or the lack of knowledge of the concepts employed in the UPSIT.
The UPSIT scores measured before surgery (22.5 ± 0.5) were lower than the values obtained in a sample of healthy subjects, who had similar ethnic background to the patient reported here [8]. The smell scores improved after surgery (33 ± 1). The improvement observed in olfactory function was significant (t = 21.0000; df = 1; P = 0.03). The PIT score was within normal limits before (39/40) and after (39/40) surgical procedure (Fig. 4).
 Click for large image | Figure 4. Smell function. Scores of the olfactory function measured by the UPSIT before (BS) and after surgery (AS). Note the significant improvement of the smell function found AS (see text for details). Bars: standard deviation. |
Surgical procedure
Removal of adrenal mass was done using laparoscopic adrenalectomy by lateral transabdominal approach [9]. The tumor was found to be encapsulated, firm, lightly yellowed, and slightly lobulated. The mass had a 4 cm diameter, and weighed 87 g. The cut surface was yellowed ruling out lipofuscin pigment. No perioperatory complications were reported during 5 days of follow-up. However, notable improvements of autonomic, nociceptive, and olfactory function were found after mass removal. Patient was lost after being discharged from the hospital.
| Discussion | ▴Top |
We have presented a unique patient who had complaints in apparently unrelated morphologic and physiologic bodily regions. However, novel neurobiological commonalities allow us to definitively explain the medical ailments of our patient. More noteworthy, this case opens a new window to understand deregulation of nociception, blood pressure, smell function linked to novel SPN and CGA biomarkers.
In clinical grounds, the suspicion on the pancreatic involvement at patient’s admission was difficult to sustain due to the multisystemic involvement and the normal laboratory values. Pain surfacing as acute pancreatitis-like syndrome in the presence of chaotic clinical findings similar to those found in our case may be the first manifestation of a pheochromocytoma (PCC) [10, 11], a catecholamine-secreting tumor, originating within the adrenal medullary chromaffin system [12]. However, the levels of catecholamines in 24-h urine collection, specifically metanephrines [13], were normal in our case. The classical “six Ps”, namely pain (headache), pressure (arterial hypertension), perspiration, palpitation, pallor, and paroxysm, which are classical clinical biomarkers in PCC [14], were also lacked in our case. Moreover, the “rule of 10s” also reported in PCC, namely, 10% extra-adrenal, 10% bilateral, 10% malignant and 10% familial, did not apply to our patient either [15, 16]. Since no hypervascularization was found on arterial-phase CTs, the diagnosis of PCC was completely abandoned. Lastly, the lack of thyroid and parathyroid involvement made multiple endocrine neoplasia diagnosis unlikely as well [17] (Table 1).
 Click to view | Table 1. Summary of the Main Glands and Cells Involved in the Different Types of Multiple Endocrine Neoplasia |
Macroscopically speaking, a “mass effect” diagnosis from the adrenal medulla could simplistically be done to explain the autonomic dysfunction presented by our patient. It is widely known that when an adrenal mass appears, it induces alterations elsewhere in the adrenal gland, being the adrenal medulla secondarily; as a result, related symptoms appear. However, the normal metanephrine values found in our case indicated that the adrenal medulla of our patient was not compromised. A conclusive way for identifying macroscopic abnormalities of the adrenal gland is to perform contrast-enhanced CT imaging of the abdomen [18]. The radiological imaging clearly showed a large adrenal mass [19], which fulfilled the radiological and pathological criteria of similar masses classified as adenoma. Noteworthy, the adrenal mass found in this case was larger in diameter compared to frequently discovered adrenocortical adenomas, usually measuring less than 3 cm [20]. Although the mass may be incriminated as responsible for the chaotic clinical picture of our patient, a more elaborate explanation could be worked out at this time.
Mixed cortical adenomas, the benign tumors most frequently found in the adrenal cortices, are often associated with unclear clinical presentations, similar to the signs and symptoms reported here [21]. It has to be remarked that the mentioned adenomas often mimic PCC [21, 22] (Table 2). Noteworthy, tumor markers such as SNP and CGA, found in our patient, have also been reported in mixed cortical adenomas [19]. However, associations between these nanobiomarkers, pain, arterial hypertension and smell dysfunction had never been reported.
 Click to view | Table 2. Frequency of Tumors Located at the Adrenal Gland |
SNP is a glycoprotein found in small and medium diameter neurons and neuroendocrine cells. It is an essential component of synaptic vesicles [23] that play a key role in synaptic transmission [24]. This protein mediates inflammatory pain in rats, and is critical in pain hypersensitization [24]. Such nociceptive regulation is controlled by cyclin-dependent kinase 5 acting on presynaptic SNP in the spinal cord dorsal horn neurons that modulates abdominal nociception [24]. Noteworthy, increased levels of SNP in spinal cord neurons correlated with nociception measures, which lowered when this protein was inhibited [24]. Altered SNP found in the ileum of guinea pigs [25] is downregulated by alpha-7 subunit of the nicotinic cholinergic receptor [26].
Germane to acetylcholine receptors involvement, alpha-3 nicotinic receptor [27], regulates CGA, a protein stored and co-released with catecholamines [28]. CGA potentiated the visceromotor response to the nociceptive action of capsaicin which modulates C-fiber activity [29, 30]. These findings were due to the action of CGA on spinal neuronal transmission. In rats, CGA was significantly increased in saliva of rats exposed to chronic pain [31]. Humans diagnosed with burning mouth syndrome also showed significant higher levels of CGA measured in saliva [32]. When neuroendocrine neoplasm like the one reported here appears, secretion of autocrine growth factors through voltage-sensitive L-type calcium channels [33] is implicated in the aberrant spinal processing of nociception mediated by nanoproteins such as CGA. To remark, CGA-associated function and dysfunction is mediated at the brainstem level, mainly over a region that encircles the nucleus of the tractus solitarius [34], which is strongly synaptophysin reactive [35], and it is heavily involved in autonomic regulation under normal conditions [36]. These mechanisms are paradoxically altered under pathological and stressful situations [37-39].
The aforementioned micro-pathophysiological mechanisms involved in autonomic function and nociception processes follow neural pathways other than those modulated by the classical medications used to treat these anomalies [40]. These facts would explain why the symptoms of our patient were resistant to the classical medications administered as well as why they went away only when the adrenal tumor was removed. These statements are further supported by descriptions done elsewhere on patient who was initially misdiagnosed as having ependymoma that resulted to be a paraganglioma, after the histological results of the mass removed were rechecked [41]. Interestingly, this tumor was positive for SNP and CGA [41]. Also, this patient had clinical symptoms very similar to those found in our patient, including chaotic arterial hypertension and altered nociception resistant to classical medications [41]. Noteworthy, the symptoms exhibited by the patient reported by Hong et al [41] normalized “immediately after resection of the tumor”. Smell function was not reported in this patient, nor has been studied in any other patient having dysfunctional any of the molecular biomarkers aforementioned.
In light of the discussed, we found that smell function is relevant in our patient for a number of reasons. First, SNP and CGA are found in olfactory pathways of healthy animals and humans [42, 43]. Second, olfaction is heavily regulated by acetylcholine neural transmission, including the alpha 3 and alpha 7 subunits, which modulate SNP and CGA function [26, 27, 44, 45]. Third, should the nanocholinergic machinery become altered, then olfactory disorders appear [8]. Fourth, unquantified smell dysfunction has been reported from patients harboring synaptophysin and CGA dysfunction associated nasal dysfunction [42, 46, 47]. Fifth, olfactory disorders have been detected by patients suffering neural disorders-associated cholinergic neurotransmission days before their actual disease surfaced [48, 49]. Sixth, quick and impressive plastic changes of olfactory function following clinical improvement similar to the trend reported here have been found in disorders associated nanocholinergic dysfunction [48, 49]. Seventh, the smell function in our patient was impaired before mass removal, regaining the olfactory function after surgical procedure, all of which was reflected in the UPSIT scores. Of note, PIT scores were within the normal range, reflecting a good understanding of the cognitive elements of the UPSIT.
Altogether, these findings support that olfactory dysfunction may well be a novel, “unclassical” and early biomarker for “classical” disorders such as pain and hypertension originated from aberrant function of nanomolecules such as SNP and CGA located in the adrenal gland, or elsewhere in the body. Lastly, a novel way to describe findings found in a single patient is presented that may re-orient current practices of reporting biomedical case reports.
| References | ▴Top |
- Guasti L, Zanotta D, Petrozzino MR, Grimoldi P, Diolisi A, Garganico D, Gaudio G, et al. Relationship between dental pain perception and 24 hour ambulatory blood pressure: a study on 181 subjects. J Hypertens. 1999;17(12 Pt 2):1799-1804.
doi pubmed - Guasti L, Gaudio G, Zanotta D, Grimoldi P, Petrozzino MR, Tanzi F, Bertolini A, et al. Relationship between a genetic predisposition to hypertension, blood pressure levels and pain sensitivity. Pain. 1999;82(3):311-317.
doi - Northcutt BG, Raman SP, Long C, Oshmyansky AR, Siegelman SS, Fishman EK, Johnson PT. MDCT of adrenal masses: Can dual-phase enhancement patterns be used to differentiate adenoma and pheochromocytoma? AJR Am J Roentgenol. 2013;201(4):834-839.
doi pubmed - Ren R, Guo M, Sneige N, Moran CA, Gong Y. Fine-needle aspiration of adrenal cortical carcinoma: cytologic spectrum and diagnostic challenges. Am J Clin Pathol. 2006;126(3):389-398.
doi pubmed - Ferrell-Torry AT, Glick OJ. The use of therapeutic massage as a nursing intervention to modify anxiety and the perception of cancer pain. Cancer Nurs. 1993;16(2):93-101.
doi - Beuschlein F, Schulze E, Mora P, Gensheimer HP, Maser-Gluth C, Allolio B, Reincke M. Steroid 21-hydroxylase mutations and 21-hydroxylase messenger ribonucleic acid expression in human adrenocortical tumors. J Clin Endocrinol Metab. 1998;83(7):2585-2588.
doi - Vesga BE, Sanabria CL, Leon-S FE. Autonomic modulation of heart frequency and blood pressure. Acta Neurol Col. 2000;16:112-120.
- Leon-Sarmiento FE, Leon-Ariza JS, Prada DG, Leon-Ariza DS. Chemosensory disturbances-associated nanocholinergic dysfunction: The case of, not only, myasthenia gravis. J Neurol Sci. 2015;356(1-2):5-6.
doi pubmed - Toniato A, Piotto A, Pagetta C, Bernante P, Pelizzo MR. Technique and results of laparoscopic adrenalectomy. Langenbecks Arch Surg. 2001;386(3):200-203.
doi pubmed - Dora JM, Siqueira DR, Meyer EL, Punales MK, Maia AL. Pancreatitis as the first manifestation of multiple endocrine neoplasia type 2A. Arq Bras Endocrinol Metabol. 2008;52(8):1332-1336.
doi pubmed - Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942.
doi pubmed - Donckier JE, Michel L. Phaeochromocytoma: state-of-the-art. Acta Chir Belg. 2010;110(2):140-148.
pubmed - van Berkel A, Lenders JW, Timmers HJ. Diagnosis of endocrine disease: Biochemical diagnosis of phaeochromocytoma and paraganglioma. Eur J Endocrinol. 2014;170(3):R109-119.
doi pubmed - Sridhar S, Parghi V. An unusual case of paraganglioma. Indian J Endocrinol Metab. 2013;17(6):1124-1126.
doi pubmed - Conzo G, Pasquali D, Colantuoni V, Circelli L, Tartaglia E, Gambardella C, Napolitano S, et al. Current concepts of pheochromocytoma. Int J Surg. 2014;12(5):469-474.
doi pubmed - Madani R, Al-Hashmi M, Bliss R, Lennard TW. Ectopic pheochromocytoma: does the rule of tens apply? World J Surg. 2007;31(4):849-854.
doi pubmed - Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med. 2011;13(9):755-764.
doi pubmed - Blake MA, Kalra MK, Maher MM, Sahani DV, Sweeney AT, Mueller PR, Hahn PF, et al. Pheochromocytoma: an imaging chameleon. Radiographics. 2004;24(Suppl 1):S87-99.
doi pubmed - Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339.
doi pubmed - Tian L, Dong J, Mo YX, Cui CY, Fan W. Adrenal cortical adenoma with the maximal diameter greater than 5 cm: can they be differentiated from adrenal cortical carcinoma by CT? Int J Clin Exp Med. 2014;7(10):3136-3143.
pubmed - Lau SK, Chu PG, Weiss LM. Mixed cortical adenoma and composite pheochromocytoma-ganglioneuroma: an unusual corticomedullary tumor of the adrenal gland. Ann Diagn Pathol. 2011;15(3):185-189.
doi pubmed - Copo J, Savio A, Soliva R, Recio H. Feocromocitoma: diagnostico y resultados del tratamiento uir gico. Rev Cubana Cirugia. 2002;41:98-103.
- Ma K, Huang Z, Ma J, Shao L, Wang H, Wang Y. Perlecan and synaptophysin changes in denervated skeletal muscle. Neural Regen Res. 2012;7(17):1293-1298.
pubmed - Zhang HH, Zhang XQ, Wang WY, Xue QS, Lu H, Huang JL, Gui T, et al. Increased synaptophysin is involved in inflammation-induced heat hyperalgesia mediated by cyclin-dependent kinase 5 in rats. PLoS One. 2012;7(10):e46666.
doi pubmed - Sharrad DF, Gai WP, Brookes SJ. Selective coexpression of synaptic proteins, alpha-synuclein, cysteine string protein-alpha, synaptophysin, synaptotagmin-1, and synaptobrevin-2 in vesicular acetylcholine transporter-immunoreactive axons in the guinea pig ileum. J Comp Neurol. 2013;521(11):2523-2537.
doi pubmed - Unger C, Svedberg MM, Schutte M, Bednar I, Nordberg A. Effect of memantine on the alpha 7 neuronal nicotinic receptors, synaptophysin- and low molecular weight MAP-2 levels in the brain of transgenic mice over-expressing human acetylcholinesterase. J Neural Transm. 2005;112(2):255-268.
doi pubmed - Sahu BS, Mohan J, Sahu G, Singh PK, Sonawane PJ, Sasi BK, Allu PK, et al. Molecular interactions of the physiological anti-hypertensive peptide catestatin with the neuronal nicotinic acetylcholine receptor. J Cell Sci. 2012;125(Pt 9):2323-2337.
doi pubmed - Kaiserova M, Vranova HP, Stejskal D, Mensikova K, Kanovsky P. Cerebrospinal fluid levels of chromogranin A in the treatment-naive early stage Parkinson's disease: a pilot study. J Neural Transm. 2013;120(11):1559-1563.
doi pubmed - Leon SF, Arimura K, Suwazono S, Arimura Y, Osame M. The effects of shounousui on the three responses of the blink reflex in man. Muscle Nerve. 1997;20(1):110-112.
doi - Aira Z, Barrenetxea T, Buesa I, Gomez-Esteban JC, Azkue JJ. Synaptic upregulation and superadditive interaction of dopamine D2- and mu-opioid receptors after peripheral nerve injury. Pain. 2014;155(12):2526-2533.
doi pubmed - Yoneyama S, Sunagawa M, Honda Y, Ikemoto H, Suga H, Iwamoto T, et al. Changes in the stress marker salivary chromogranin A, accociated with acute and chronic pain. Showa Graduate J. 2013;2:85-90.
- Chieko S-H, Soh I, Yoshida A, Awano S, Anan H, Ansai T. Salivary levels of cortisol and chromogranin A in patients with burning mouth syndrome: A case-control study. Open J Stomatology. 2013;3:39-43.
doi - Kraszewski S, Drabik D, Langner M, Ramseyer C, Kembubpha S, Yasothornsrikul S. A molecular dynamics study of catestatin docked on nicotinic acetylcholine receptors to identify amino acids potentially involved in the binding of chromogranin A fragments. Phys Chem Chem Phys. 2015;17(26):17454-17460.
doi pubmed - Avolio E, Mahata SK, Mantuano E, Mele M, Alo R, Facciolo RM, Talani G, et al. Antihypertensive and neuroprotective effects of catestatin in spontaneously hypertensive rats: interaction with GABAergic transmission in amygdala and brainstem. Neuroscience. 2014;270:48-57.
doi pubmed - Rao H, Pio J, Kessler JP. Postnatal development of synaptophysin immunoreactivity in the rat nucleus tractus solitarii and caudal ventrolateral medulla. Brain Res Dev Brain Res. 1999;112(2):281-285.
doi - Tota B, Angelone T, Cerra MC. The surging role of Chromogranin A in cardiovascular homeostasis. Front Chem. 2014;2:64.
doi pubmed - Loh YP, Cheng Y, Mahata SK, Corti A, Tota B. Chromogranin A and derived peptides in health and disease. J Mol Neurosci. 2012;48(2):347-356.
doi pubmed - Helle KB, Corti A. Chromogranin A: a paradoxical player in angiogenesis and vascular biology. Cell Mol Life Sci. 2015;72(2):339-348.
doi pubmed - Vaingankar SM, Li Y, Biswas N, Gayen J, Choksi S, Rao F, Ziegler MG, et al. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J Hypertens. 2010;28(4):817-825.
doi pubmed - Orban-Gyapai O, Raghavan A, Vasas A, Forgo P, Hohmann J, Shah ZA. Flavonoids isolated from Rumex aquaticus exhibit neuroprotective and neurorestorative properties by enhancing neurite outgrowth and synaptophysin. CNS Neurol Disord Drug Targets. 2014;13(8):1458-1464.
doi pubmed - Hong JY, Hur CY, Modi HN, Suh SW, Chang HY. Paraganglioma in the cauda equina. A case report. Acta Orthop Belg. 2012;78(3):418-423.
pubmed - Utsuki S, Kawano N, Oka H, Shimizu S, Sagiuchi T, Saegusa H, Fujii K, et al. Olfactory neuroepithelioma arising from the olfactory placode. Clin Neuropathol. 2000;19(1):7-12.
pubmed - Johnson BA, Woo CC, Ninomiya-Tsuboi K, Leon M. Synaptophysin-like immunoreactivity in the rat olfactory bulb during postnatal development and after restricted early olfactory experience. Brain Res Dev Brain Res. 1996;92(1):24-30.
doi - Doty RL. The olfactory system and its disorders. Semin Neurol. 2009;29(1):74-81.
doi pubmed - Mahata SK, Mahata M, Fung MM, O'Connor DT. Catestatin: a multifunctional peptide from chromogranin A. Regul Pept. 2010;162(1-3):33-43.
doi pubmed - Mohebbi AR, Daneshi A, Emami AR. Nasopharyngeal neuroendocrine carcinoma: a case report. Ear Nose Throat J. 2008;87(12):E14.
pubmed - Pizzoni C, Sarandria C, Pierangeli E. Clear-cell meningioma of the anterior cranial fossa. Case report and review of the literature. J Neurosurg Sci. 2009;53(3):113-117.
pubmed - Leon-Sarmiento FE, Leon-Ariza DS, Doty RL. Dysfunctional chemosensation in myasthenia gravis: a systematic review. J Clin Neuromuscul Dis. 2013;15(1):1-6.
doi pubmed - Leon-Sarmiento FE, Bayona EA, Bayona-Prieto J, Osman A, Doty RL. Profound olfactory dysfunction in myasthenia gravis. PLoS One. 2012;7(10):e45544.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


