| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 7, Number 6, June 2016, pages 234-238
Excessive Uterine Bleeding in a Non-Compliant Patient With Profound Hypothyroidism: A Case Report and Review of the Literatures
Ben-Jiang Ma
Department of Endocrinology, Sacred Heart Hospital, 1549 Airport Blvd, Pensacola, FL 32504, USA. Current address: IPC Healthcare Southeast Florida, 1540 International Parkway, Lake Mary, FL 32746, USA
Manuscript accepted for publication May 03, 2016
Short title: Profound Hypothyroidism and Menorrhagia
doi: http://dx.doi.org/10.14740/jmc2502w
| Abstract | ▴Top |
Studies suggest that dysthyroidism is associated with disturbances in female menstruation with hypothyroidism being associated with abnormal uterine bleeding. We report a case of excessive uterine bleeding due to severe hypothyroidism. The patient was a 35-year-old female with a history of papillary thyroid cancer, status post total thyroidectomy and subsequent two times of radioactive iodine treatment for residual thyroid cancer. She presented to emergency room two times in 4 days for persistent heavy vaginal bleeding. Her complete blood count (CBC) upon admission showed hemoglobulin (HgB) of 7.0 g/dL and hematocrit (HCT) of 20.0%. The patient admitted that she had not taken levothyroxine (LT4) for at least 4 weeks. The thyroid function test showed elevated thyroid-stimulating hormone (TSH) level of 74.71 mIU/L and decreased free T4 level of 0.55 ng/dL (reference range is 0.34 - 5.60 mIU/L and 0.58 - 1.64 ng/dL, respectively). The patient was given two units of packed red blood cell transfusion to correct the hemorrhagic anemia and then was given 25 mg of conjugated estrogen (Premarin) intravenously (IV) to control the bleeding. She was discharged on oral medroxyprogesterone acetate (Provera) and LT4 and was asked for regular follow-ups. This is an uncommon but representative case of acute menstrual blood loss anemia caused by profound hypothyroidism. The disturbance of hypothalamus-pituitary-ovarian axis due to the severe hypothyroidism is likely the major etiology for the excessive dysfunctional uterine bleeding.
Keywords: Hypothyroidism; Dysfunctional uterine bleeding; Menorrhagia; Anemia; Pituitary glycoprotein hormones
| Introduction | ▴Top |
It has long been a documented phenomenon that thyroid dysfunctions, both hyper- and hypothyroidism, are associated with menstrual disturbances. Although remarkable discrepancy exists, hyperthyroidism is more associated with secondary amenorrhea or oligomenorrhea while hypothyroidism is associated with polymenorrhea or menorrhagia [1, 2]. The mechanism of hypothyroidism to cause dysfunctional uterine bleeding (DUB) is multifactorial, including impairment of hemostasis and neuro-endocrine dysfunction.
Here we report a case of heavy uterine bleeding in a profound but non-myxedematous hypothyroid patient due to medication non-compliance and the exploration of possible mechanisms by review of the literatures.
| Case Report | ▴Top |
The patient was a 35-year-old G2 P1-0-1-1 female with a history of papillary thyroid cancer, status post total thyroidectomy in 2005 followed by subsequent two times of radioactive iodine treatments, and a pertinent obstetrical history of preeclampsia that required cesarean section in 2004 and a spontaneous abortion in 2007. She presented to the emergency room (ER) with worsening heavy menstrual bleeding for the past 2 weeks. She had an ER visit 4 days ago for the same complaint. The patient was ruled out pregnancy by urine human chorionic gonadotropin (hCG) testing. Her complete blood count (CBC) in the prior visit (day -4) showed hemoglobulin (HgB) of 9.2 g/dL, hematocrit (HCT) of 27.3%, and elevated red-cell distribution width (RDW) of 21.8% (Table 1). She was discharged with tranexamic acid (Lysteda) 650 mg daily but she did not take it. Her bleeding had continued with passage of golf-ball sized clots along with mild to moderate abdominal pain and dizziness when moving. She reported to change 3 - 5 pads per day. In the current ER visit (day 0), her CBC showed much lower HgB and HCT (H/H) of 7.0 g/dL and 20.0%, respectively (Table 1). Endovaginal ultrasound showed a thin lining of endometrium with a composite thickness of 1.2 mm and the presence of bilateral functional ovarian cysts.
 Click to view | Table 1. Complete Blood Count |
Further workup showed markedly elevated thyroid-stimulating hormone (TSH) level of 74.71 mIU/L and decreased free T4 level of 0.55 ng/dL (reference range is 0.34 - 5.60 mIU/L and 0.58 - 1.64 ng/dL, respectively; Table 2). Pituitary hormones including follicular-stimulating hormone (FSH) and luteinizing hormone (LH) were notably low (2.9 and 5.6 mIU/mL, respectively) for the time point of menstruation (Table 2).
 Click to view | Table 2. Hormone Levels |
The patient was transfused two units of packed red blood cell (PRBC), which raised the H/H to 10.0 g/dL and 28.4% in the next day’s (day 1) CBC lab (Table 1 and Fig. 1). She was then given 25 mg of conjugated estrogen (Premarin) intravenously (IV) once to control the bleeding and oral medroxyprogesterone acetate (Provera) 10 mg daily.
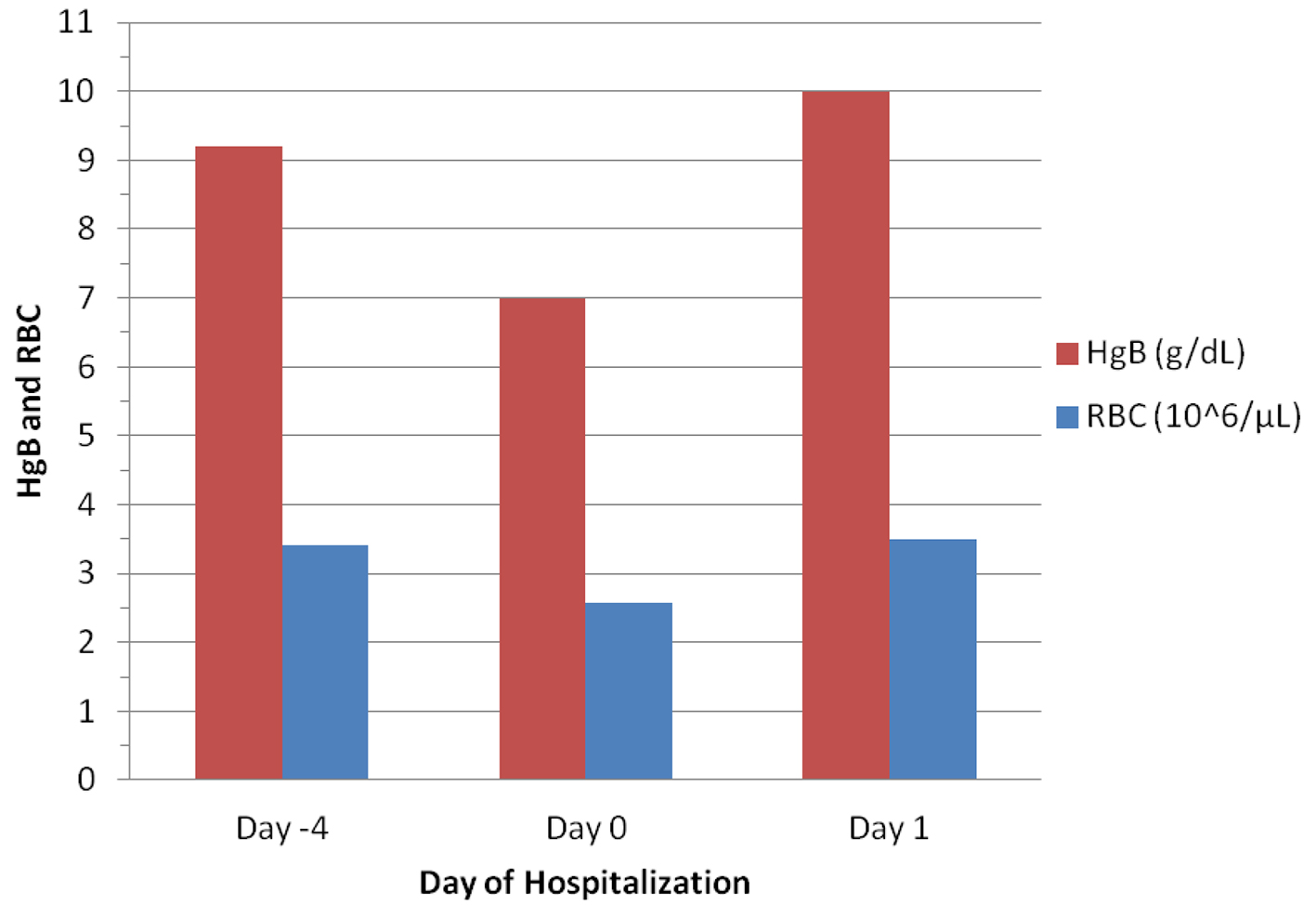 Click for large image | Figure 1. Changes in hemoglobulin (HgB) and red blood cells (RBC) before and after blood transfusion (given on Day 0). |
The patient admitted that she had been off levothyroxine (LT4, supposedly 150 µg daily) for at least 4 weeks. Even when she did, she usually took it with hypertension medicines carvedilol and lisinopril together in the morning.
The patient denied history of easy bruising or family history of bleeding disorders. She did not use hormonal contraceptives. The physical examination revealed a neurologically intact patient in flat affect with stable vital signs. There were no petechiae, purpura, ecchymosis, or non-pitting edema.
| Discussion | ▴Top |
Abnormal uterine bleeding and related terminology
Normal menstrual cycle is 21 - 35 days with menses period of 3 - 7 days and average blood loss of 30 - 60 mL [3-5]. The normal menstrual cycle consists of three phases: the first is follicular phase, in which the elevated FSH causes a dominant follicle to mature and produce estrogen in the granulosa cells. The increased estrogen causes the cease of menstrual flow and the proliferation of endometrium linings, and the positive feedback promotes LH surge, resulting in the second phase - ovulation. In the third luteal phase, elevated progesterone produced by the corpus luteum halts proliferation of the endometrium and promotes its differentiation; when the progesterone level declines, the endometrial lining sheds, and the menstruation results [4].
Abnormal uterine bleeding (AUB) is an umbrella definition for any irregular (in terms of regularity, frequency, duration, and volume of blood loss) menstruation with or without identifiable causes [4]. It has long been debated among the practitioners on the accuracy and the content of concept of AUB [3, 6]. The denotation of AUB is different among physicians between US and internationals [3]. Since 2005, a panel of international specialists have been working on the causes of AUB based on the structural and/or functional abnormalities and reached a consensus of PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified) classification system, which was approved by the Executive Board of International Federation of Gynecology and Obstetrics (FIGO) in 2011 [7, 8].
Although no longer recommended by FIGO [8], the nomenclatures such as DUB and menorrhagia are still frequently used. The widely accepted definition for DUB is abnormal uterine bleeding that occurs in the absence of pelvic pathology or identifiable medical illness. DUB is considered the most common cause of AUB but remains a diagnosis of exclusion [9, 10]. DUB is classified as ovulatory and anovulatory [4]; in the US, DUB usually refers anovulatory [3].
Anovulatory DUB is more common and is usually associated with reproductive endocrine (hypothalamus-pituitary-ovary axis) dysfunction. It results from the excessive or unopposed estrogens that disrupt the normal cyclic pattern of ovulatory hormonal stimulation of the endometrial lining [3, 4]. It is thought to be associated with either estrogen breakthrough/withdrawal or progesterone breakthrough [4].
Ovulatory DUB is predominantly associated with decreased endometrial vasoconstriction and vascular haemostatic plug formation, leading to defective control of the volume of blood which is lost during menstruation. Ovulatory DUB may be associated with disturbed angiogenesis, fragile vessels and defective haemostatic processes [3, 4].
Menorrhagia, although not clearly defined, is a term used for over 300 years in the literatures [6]. It is usually considered ovulatory type of DUB, which is clinically defined as excessive flow (greater than 80 mL of total blood loss) and prolonged duration (more than 7 days) at regular cycle intervals [3-5]. In practice, however, it is also used in anovulatory DUB for convenience to describe the severe bleeding. In addition, the volume of blood loss is usually assessed clinically, i.e., the number of pads changed, or the degree of anemia [5, 10].
Profound hypothyroidism
Hypothyroidism is defined as decreased thyroxines (T4 and T3) and elevated TSH levels (usually greater than 5.0 mIU/L), and the upper limit of latter is still controversial and is subject to age-specific definition [11]. The etiology of hypothyroidism is broad [12]. This patient is iatrogenically hypothyroid due to total thyroidectomy and subsequent two times of radioactive iodine ablation for the treatment of thyroid cancer. In most cases, hypothyroid patient can be successfully treated with LT4 [12]. However, issues with treatment adherence have been reported to be the most common cause of treatment failure for hypothyroidism [13]. The condition of this particular medical non-compliance is specifically termed “pseudomalabsorption of LT4” [14, 15].
Hypothyroidism is associated with multi-organ dysfunctions, including cardiovascular, neuromuscular, gastrointestinal and reproductive systems [16, 17]. Severe hypothyroidism may cause failure of ovulation secondary to hypogonadism. If, however, the ovulation and conception do occur, the chances of abortions, stillbirths, or prematurity are much higher [17]. Secondary to medication non-compliance, this patient had multiple episodes of severe hypothyroidism with various complications. During the months of September and October in 2007, her TSH was elevated from 70 to over 100 mIU/L (data not shown). In those times, she was hospitalized for a spontaneous abortion (SAB), and an acute decompensated congestive heart failure (CHF) with elevated b-type natriuretic peptide (BNP) levels (peaked > 1,100 pg/mL, data not shown) and non-ischemic cardiomyopathy with ejection fraction (EF) of 29%.
In this admission, despite the markedly elevated TSH level, her free T4 was moderately reduced and free T3 level, the bioactive form of thyroxine, was still in the low normal range (Table 2). This explains the minimal subjective symptoms of hypothyroidism and the absence of myxedema, a severe form of hypothyroidism [12].
Severe hypothyroidism and DUB
As mentioned above, the causes of DUB are broad, including local structural abnormality, pelvic pathology, and systemic diseases [3, 5]. It was well documented that hypothyroidism is associated with polymenorrhea or menorrhagia [1]; profound hypothyroidism has been reported to cause life-threatening menorrhagic anemia [18]. There is accumulating evidence on the cause-effect relationship between hypothyroidism and menorrhagia; the treatment of hypothyroidism has been reported to ameliorate the menstrual blood loss [19].
The mechanism of hypothyroidism to cause excessive uterine bleeding is multifactorial. Physiologically, the platelet plays an important role in hemostasis during menstruation [3]. It is recognized that overt or even subclinical hypothyroidism can not only cause anemia, but also the platelet dysfunction, particularly the acquired von Willebrand syndrome [20, 21]. In addition, the meta-analysis by Squizzato et al showed that overt dysthyroidism disturbs the coagulation-fibrinolytic balance with hypocoagulable state associated with hypothyroidism and a hypercoagulable state with hyperthyroidism [22].
Emerging evidence supports close physiologic and pathologic interactions between hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-ovarian (HPO) axis. The presence of thyroid hormone receptors on the ovaries suggests the influence of reproductive function by thyroid function, while the estrogens may exert its effect at the higher levels of the HPT axis [23]. Studies by Sower et al suggest the existence of a primitive, overlapping yet functional HPO and HPT endocrine systems by sharing the pituitary glycoprotein hormone/glycoprotein hormone receptor systems as a link between the neuro-hormonal and peripheral control of reproductive system [24].
Pituitary hormones including LH, FSH, TSH and the placenta-derived chorionic gonadotropin (hCG) are glycoproteins that consist of two strong non-covalently bond components, the alpha unit and beta unit. The alpha unit is common to all these hormones while the beta unit is specific to individual hormone [25]. The beta-subunits are responsible for receptor binding on target cells whereas the alpha-subunits are essential for receptor transactivation through G-protein-coupled receptors. Physiologically, synthesis and secretions of alpha- and beta-chain are highly coupled with only small amounts of free monomeric subunits. This coordinated production of intact hormones may be disturbed and disproportionate quantities of free alpha-subunits are secreted in pituitary adenomas, being most commonly associated with gonadotroph- or thyrotroph-derived tumors [26]. Hypothalamic gonadotropin releasing hormone (GnRH) and thyrotropin-releasing hormone (TRH) or their agonists/antagonists can also alter the alpha subunit level [25].
In severe hypothyroid patient, the TSH is markedly increased due to the weakened or absence of negative feedback from thyroxines (T4 and T3). The hypersecretion of TSH may result in hyperplasia of thyrotropes in the pituitary gland, as recently reported by Doyle et al in a case of “pseudomalabsorption of LT4” with much elevated TSH (> 700 mIU/L) [27]. The mass effect of pituitary hyperplasia can suppress the secretion of LH [14, 15, 27]. Due to the same mechanism of lack of feedback suppression, the hypophyseal TRH is also expected to be elevated, which increases the secretion of both TSH and prolactine (PRL) levels; the hyperprolactinemia will in turn suppress the LH [1]. TSH has a small FSH- and LH-like effect. Depending on the degree of disturbance of these upper level hormones, the estrogen level may remain elevated inappropriately and unopposed, which results in anovulatory DUB [1]. In addition, the spillover of the free alpha subunit pituitary glycoprotein may have regulatory effect on HPT/HPO axes as well as the uterus. All these abnormalities could contribute to DUB or menorrhagia.
Therefore, the causes of menorrhagia are multifactorial, and multiple etiologies can coexist in one case. This patient had no personal or family history of bleeding disorders or hemoglobinopathy. She did have some degree of microcytic, hypochromic anemia with markedly elevated RDW, about 22.0% before transfusion (Table 1). Although the acquired platelet dysfunction could not be ruled out, her menorrhagia was more likely secondary to the neuro-endocrine disturbances due to the elevated TSH along with relatively low LH and FSH levels (Table 2). Since TSH has some FSH and LH properties, it may stimulate the estrogen production. The unopposed estrogen not only negatively inhibits FSH and LH but also results in endometrial hyperplasia that eventually lead to heavy breakthrough bleeding [3, 4, 9]. The relatively low LH and FSH could also be a result of chronically elevated TSH caused by the hyperplasia of thyrotrophs that exerted the mass effect on FSH and LH-secreting pituitary cells, although no MRI study was obtained to confirm this hypothesis.
Treatment and management of anemia due to excessive DUB
Treatment of acute blood loss anemia is directed by the patient’s clinical presentation and pertinent laboratory profiles and should follow the rule of ABC (airway, breathing, circulation). In the emergent setting, hemodynamic stability is of great importance [5]. In this patient, her nadir of hemoglobulin was 7.0 g/dL, which was improved to 10.0 g/dL with two units PRBC (Table 1 and Fig. 1). Acute treatment with IV premarin significantly reduced the bleeding by the elevated serum estrogen level (Table 2). The mechanisms include induction of capillary vasospasm, reducing capillary permeability, upregulation of levels of fibrinogen, factor IV, and factor X and enhancement of platelet aggregation. It also upregulates progesterone receptors, making subsequent treatment with progestins more effective [5, 28]. Long-term treatment is based on the underlying etiology of DUB. In this patient, she had no means of contraception. Therefore, long-acting progestin, medroxyprogesterone acetate (Provera) is of wise choice [5].
For the treatment of hypothyroidism, the patient was restarted LT4 150 µg daily and was asked to follow up in 4 - 6 weeks. A lengthy education was given to the patient about the importance of medication compliance to prevent complications from severe hypothyroidism, including SAB, DUB, and CHF [16, 17, 29]. The adherence to the treatment plan is particularly important for this patient with history of thyroid cancer, because LT4 can not only correct hypothyroidism, but also prevent recurrence of thyroid cancer [30].
Grants
None.
Disclosure
The authors have nothing to disclose.
| References | ▴Top |
- Koutras DA. Disturbances of menstruation in thyroid disease. Ann N Y Acad Sci. 1997;816:280-284.
doi pubmed - Kakuno Y, Amino N, Kanoh M, Kawai M, Fujiwara M, Kimura M, Kamitani A, et al. Menstrual disturbances in various thyroid diseases. Endocr J. 2010;57(12):1017-1022.
doi pubmed - Livingstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Hum Reprod Update. 2002;8(1):60-67.
doi pubmed - Albers JR, Hull SK, Wesley RM. Abnormal uterine bleeding. Am Fam Physician. 2004;69(8):1915-1926.
pubmed - Apgar BS, Kaufman AH, George-Nwogu U, Kittendorf A. Treatment of menorrhagia. Am Fam Physician. 2007;75(12):1813-1819.
pubmed - Fraser IS, Critchley HO, Munro MG. Abnormal uterine bleeding: getting our terminology straight. Curr Opin Obstet Gynecol. 2007;19(6):591-595.
doi pubmed - Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3-13.
doi pubmed - Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383-390.
doi pubmed - Oriel KA, Schrager S. Abnormal uterine bleeding. Am Fam Physician. 1999;60(5):1371-1380; discussion 1381-1372.
- Maness DL, Reddy A, Harraway-Smith CL, Mitchell G, Givens V. How best to manage dysfunctional uterine bleeding. J Fam Pract. 2010;59(8):449-458.
pubmed - Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95(2):496-502.
doi pubmed - Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615, v.
doi pubmed - Hanna FW, Lazarus JH, Scanlon MF. Controversial aspects of thyroid disease. BMJ. 1999;319(7214):894-899.
doi pubmed - Ogawa D, Otsuka F, Mimura U, Ueno A, Hashimoto H, Kishida M, Ogura T, et al. Pseudomalabsorption of levothyroxine: a case report. Endocr J. 2000;47(1):45-50.
doi pubmed - Eledrisi MS, Szymajda A, Alshanti M, Urban RJ. Noncompliance with medical treatment: pseudomalabsorption of levothyroxine. South Med J. 2001;94(8):833-836.
doi pubmed - Sharma AK, Arya R, Mehta R, Sharma R. Hypothyroidism and cardiovascular disease: factors, mechanism and future perspectives. Curr Med Chem. 2013;20(35):4411-4418.
doi pubmed - Krassas GE. Thyroid disease and female reproduction. Fertil Steril. 2000;74(6):1063-1070.
doi - Moragianni VA, Somkuti SG. Profound hypothyroidism-induced acute menorrhagia resulting in life-threatening anemia. Obstet Gynecol. 2007;110(2 Pt 2):515-517.
doi pubmed - Weeks AD. Menorrhagia and hypothyroidism. Evidence supports association between hypothyroidism and menorrhagia. BMJ. 2000;320(7235):649.
doi - Manfredi E, van Zaane B, Gerdes VE, Brandjes DP, Squizzato A. Hypothyroidism and acquired von Willebrand's syndrome: a systematic review. Haemophilia. 2008;14(3):423-433.
doi pubmed - Stuijver DJ, Piantanida E, van Zaane B, Galli L, Romualdi E, Tanda ML, Meijers JC, et al. Acquired von Willebrand syndrome in patients with overt hypothyroidism: a prospective cohort study. Haemophilia. 2014;20(3):326-332.
doi pubmed - Squizzato A, Romualdi E, Buller HR, Gerdes VE. Clinical review: Thyroid dysfunction and effects on coagulation and fibrinolysis: a systematic review. J Clin Endocrinol Metab. 2007;92(7):2415-2420.
doi pubmed - Doufas AG, Mastorakos G. The hypothalamic-pituitary-thyroid axis and the female reproductive system. Ann N Y Acad Sci. 2000;900:65-76.
doi pubmed - Sower SA, Freamat M, Kavanaugh SI. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys. Gen Comp Endocrinol. 2009;161(1):20-29.
doi pubmed - Lindner J, Rivier JE, Vale WW, Pavlou SN. Regulation of pituitary glycoprotein alpha-subunit secretion after administration of a luteinizing hormone-releasing hormone antagonist in normal men. J Clin Endocrinol Metab. 1990;70(4):1219-1224.
doi pubmed - Samejima N, Yamada S, Takada K, Sano T, Ozawa Y, Shimizu T, Usui M, et al. Serum alpha-subunit levels in patients with pituitary adenomas. Clin Endocrinol (Oxf). 2001;54(4):479-484.
doi - Doyle MA, Lochnan HA. Pituitary hyperplasia: a complication of the pseudomalabsorption of thyroxine. Int J Gen Med. 2013;6:335-339.
doi pubmed - Munro MG. Dysfunctional uterine bleeding: advances in diagnosis and treatment. Curr Opin Obstet Gynecol. 2001;13(5):475-489.
doi pubmed - Mitchell JE, Hellkamp AS, Mark DB, Anderson J, Johnson GW, Poole JE, Lee KL, et al. Thyroid function in heart failure and impact on mortality. JACC Heart Fail. 2013;1(1):48-55.
doi pubmed - Wu LS, Roman SA, Sosa JA. Medullary thyroid cancer: an update of new guidelines and recent developments. Curr Opin Oncol. 2011;23(1):22-27.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


