| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 2, Number 5, October 2011, pages 222-224
Bilateral Papilledema Following Human Papillomavirus Vaccination
Marco Rossia, b, d, Chiara Bettinia, b, Caterina Paganoc
aCentre for Pharmacovigilance, University Hospital of Siena, Strada delle Scotte, 6, 53100 Siena, Italy
bTuscan Regional Centre for Pharmacovigilance, Florence, Italy
cPaediatrics and Neonatology Unit, Misericordia Hospital, Via Senese, 58100 Grosseto, Italy
dCorresponding author: Marco Rossi, E-mail address:
Manuscript accepted for publication July 29, 2011
Short title: Bilateral Papilledema
doi: https://doi.org/10.4021/jmc256w
| Abstract | ▴Top |
The human papillomavirus (HPV) vaccines have been shown to be effective in preventing pre-cancerous lesions and are regarded by health professionals as generally safe and well-tolerated. Neurological complications following immunization are extremely rare. We report an 11-year-old girl who presented with bilateral papilledema ten days after the administration of a bivalent HPV vaccine.
Keywords: Papilledema; Papillomavirus vaccines; Vaccination
| Introduction | ▴Top |
Two human papillomavirus (HPV) recombinant vaccines, a quadrivalent (types 6, 11, 16 and 18) and a bivalent vaccine (types 16 and 18) have been shown to be effective in the management of HPV by preventing vaccine subtype-related persistent infection and precancerous lesions as evidenced by numerous worldwide clinical trials involving more than 59,000 participants. Following the licensing and marketing of the vaccines, large scale immunization programs for the prevention of cervical cancer have been implemented in a number of industrialized countries, including the United States, Australia and western European countries, targeting primarily females with an age range from 9 to 17 years.
The current evidence on the safety of HPV vaccines is reassuring. The most frequently adverse events following immunization (AEFIs) reported to the United States Vaccine Adverse Event Reporting System (VAERS) were vasovagal syncope, local reactions, dizziness, nausea and headache and most of the AEFI rates were not greater than the background rates compared with other vaccines [1].
Neurological AEFIs are quite exceptional. We present a case of bilateral papilledema following the administration of a HPV bivalent vaccine reported to the Italian Pharmacovigilance System.
| Case Report | ▴Top |
An 11-year-old girl with an uneventful medical history developed diplopia and headache ten days following the second dose of bivalent HPV vaccine. Diagnosis of bilateral papilledema was made by an ophthalmologist and the patient was admitted to the pediatric unit of the local hospital. Physical examination revealed good general condition without neurological signs and symptoms.
Further ophthalmological controls showed normal visus and diplopia with paresis of the left lateral m.rectus.Bilateral papilla stasis was found on a fundus examination (Fig. 1) and a visual field test was compatible with bilateral papilledema (Fig. 2).
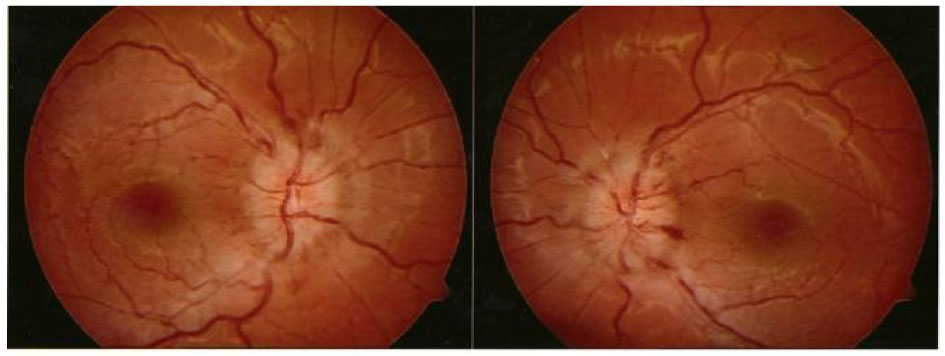 Click for large image | Figure 1. Fundus images, showing bilateral optic disk edema. |
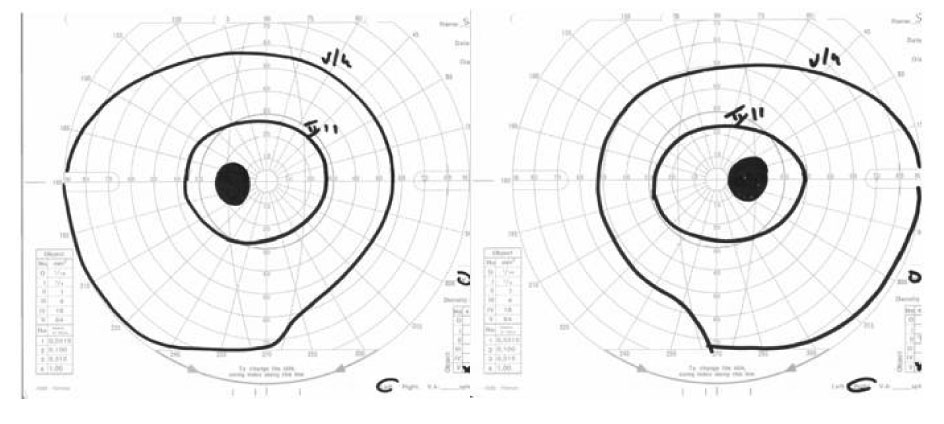 Click for large image | Figure 2. Visual field. |
Intracranial pressure estimated during lumbar puncture was in the normal range. CT scans of brain and orbits and a brain MRI with contrast medium did not show any anatomical anomaly.
Physical-chemical examination of the cerebrospinal fluid (CSF) and the analysis of the CSF protein composition did not reveal any abnormalities. Blood pressure measurements performed by 24-hours Holter monitoring were not elevated. Further laboratory tests in the normal range were: glycemic profile, cortisol, progesterone, 17-OH progesterone, DHEA-S, testosterone, aldosterone, androstenedione, FT3, FT4, TSH, C3, C4, ANA, ENA, anti-DNA antibodies. Serological markers were negative for Herpes simplex virus, Cytomegalovirus, Epstein-Barr virus, Mycoplasma pneumoniae, Treponema pallidum and Toxoplasma gondii.
After a treatment with corticosteroids and furosemide, the patient presented slow progression with complete regression of diplopia and normalization of fundus and visual field examination after approximately two months.
| Discussion | ▴Top |
Onset of bilateral and unilateral papilledema after vaccinations have been reported in the medical literature, in most cases secondary to demyelinating disorders. A PubMed search (1966 – June 2011) using the medical subject headings (MeSH) terms “papilledema” and “vaccines/adverse effects” retrieved cases of papilledema following hepatitis B [2, 3], rabies [4, 5], influenza [6] and bacillus Calmette-Guerin [7] vaccines. An on-line query on VAERS using CDC WONDER search tool results in eight cases of papilledema following quadrivalent HPV vaccine [8]. Five cases of central nervous system (CNS) demyelination [9] and one case of acute disseminated encephalomyelitis [10] following quadrivalent HPV vaccine have been described. Recently four cases of demyelinating diseases (two cases of transverse myelitis, one case of retrobulbar optic neuritis and one case of neuropraxia of the facial nerve) were diagnosed in young women aged 15 - 27 year-old with an onset ranging from seven days to one month after HPV vaccination [11].
In our case a role of the bivalent HPV vaccine in the pathogenesis of the bilateral papilledema observed can not be excluded on the basis of the temporal association, but biological plausibility is lacking. CSF biochemistry and laboratory data did not support the hypothesis of a papillitis caused by an immune-mediated reaction possibly triggered by the vaccine. Moreover, the fundus examination and the visual fields are more suggestive of papilledema than papillitis and normal visual acuity due to papillitis in the presence of grossly swollen discs is unusual. Although the clinical features (headache, papilledema, lateral rectus palsy) suggest raised intracranial pressure, in our patient the opening CSF pressure was not elevated. On the other hand, neuroimaging studies did not show evidence of intracranial mass, vascular abnormalities, obstruction of the ventricular system or optic neuropathies.
Causality between vaccines and the occurrence of neurological complications is difficult to establish from individual case reports, because in most cases it is not possible to definitely distinguish whether an observed event that follows shortly after a vaccination is causal or coincidental. To date there is no evidence for an increased risk of autoimmune demyelinating diseases following HPV vaccines. In a review of reports to VAERS following quadrivalent HPV vaccine from June 1, 2006 through December 31, 2008, Slade et al. [1] calculated a rate of 0.04 for transverse myelitis and 0.2 for Guillain-Barre syndrome (GBS) per 100,000 doses distributed, finding no evidence for disproportionate reporting of GBS after quadrivalent HPV vaccine as compared with GBS reported after other adolescent vaccines. Nevertheless, given the very low incidence of neurological AEFIs and the limitations of spontaneous reporting systems, including underreporting, unverified reports and inconsistent data quality, large case – control studies such as the ongoing study using the French PGRx®(Pharmacoepidemiological General Research) Program to assess the potential association between quadrivalent HPV vaccine and the occurrence of autoimmune/inflammatory diseases, including GBS and other demyelinatingdiseases [12], are warranted to better clarify possible neurological AEFIs following the administration of HPV vaccines.
Conflict of Interest
The authors declare that they have no conflict of interest.
| References | ▴Top |
- Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, Izurieta HS, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302(7):750-757.
pubmed doi - Berkman N, Benzarti T, Dhaoui R, Mouly P. [Bilateral neuro-papillitis after hepatitis B vaccination]. Presse Med. 1996;25(28):1301.
pubmed - Fledelius HC. Unilateral papilloedema after hepatitis B vaccination in a migraine patient. A case report including forensic aspects. Acta Ophthalmol Scand. 1999;77(6):722-724.
pubmed doi - Gupta V, Bandyopadhyay S, Bapuraj JR, Gupta A. Bilateral optic neuritis complicating rabies vaccination. Retina. 2004;24(1):179-181.
pubmed doi - Thomas C, Canton P, Raspiller A, Delaveuve J. [Papillitis, a complication of rabies vaccination]. Bull Soc Ophtalmol Fr. 1972;72(1):129-132.
pubmed - Kawasaki A, Purvin VA, Tang R. Bilateral anterior ischemic optic neuropathy following influenza vaccination. J Neuroophthalmol. 1998;18(1):56-59.
pubmed doi - Agadzi VK. BCG complications: an analysis of 36 cases. Dev Biol Stand. 1978;41:75-78.
pubmed - United States Department of Health and Human Services (DHHS), Public Health Service (PHS), Centers for Disease Control (CDC) / Food and Drug Administration (FDA), Vaccine Adverse Event Reporting System (VAERS) 1990 - last month, CDC WONDER On-line Database Accessed at http://wonder.cdc.gov/vaers.html on Jun 8, 2011.
- Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Mult Scler. 2009;15(1):116-119.
pubmed doi - Wildemann B, Jarius S, Hartmann M, Regula JU, Hametner C. Acute disseminated encephalomyelitis following vaccination against human papilloma virus. Neurology. 2009;72(24):2132-2133.
pubmed doi - Alvarez-Soria MJ, Hernandez-Gonzalez A, Carrasco-Garcia de Leon S, del Real-Francia MA, Gallardo-Alcaniz MJ, Lopez-Gomez JL. [Demyelinating disease and vaccination of the human papillomavirus]. Rev Neurol. 2011;52(8):472-476.
pubmed - Bonanni P, Cohet C, Kjaer SK, Latham NB, Lambert PH, Reisinger K, Haupt RM. A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD. Vaccine. 2010;28(30):4719-4730.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


