| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 8, Number 4, April 2017, pages 127-131
Synchronous Infrasellar and Cervical Chondroid Chordoma: A Case Report and Review of the Literature
Ilson Sepulvedaa, e, Thomas Schmidtb, Geraldine Ayresc, Paulo Verad, Felipe Fredesb
aRadiology Department, ENT-Head and Neck Surgery Service, General Hospital of Concepcion, Chile
bENT-Head and Neck Surgery Service, General Hospital of Concepcion, University of Concepcion School of Medicine, Concepcion, Chile
cPathology Department, General Hospital of Concepcion, University of Concepcion School of Medicine, Concepcion, Chile
dRadiotherapy Department, Oncology Service, General Hospital of Concepcion, Chile
eCorresponding Author: Ilson Sepulveda, Radiology Department, ENT-Head and Neck Surgery Service, General Hospital of Concepcion, San Martin Av 1436, Concepcion, Chile
Manuscript accepted for publication March 17, 2017
Short title: Synchronous Infrasellar and Cervical CHCD
doi: https://doi.org/10.14740/jmc2791w
| Abstract | ▴Top |
We report on a patient who presented in ENT clinic with diplopia accompanied by headache and vomiting. Imaging studies revealed a large expansive heterogeneous mass encroaching on the infrasellar region and cervical spine. Post-intravenous contrast administration revealed a lesion with high enhancement and involvement of the sphenoidal sinus with retroclival extension. Biopsy confirmed the presence of a chondroid chordoma. Treatment included radiation therapy and the patient is currently receiving palliative and supportive care.
Keywords: Tumor; Chondroid; Chordoma; Head; Neck; Radiotherapy; Magnetic resonance imaging; Computed tomography
| Introduction | ▴Top |
Chordomas (CDS) are malignancies that arise from cellular remnants of the embryonic notochord [1]. The ratio of males to females with chordoma is approximately 1:1, between the third and fifth decades of life [2]. In the head and neck, these tumors are locally aggressive but metastasis to the lymph nodes of the neck is rare. Imaging studies are essential for preoperative staging and surgical planning [2]. The treatment of choice is complete surgical resection and adjuvant radiotherapy [1].
| Case Report | ▴Top |
A 68-year-old female with medical history of arterial hypertension and type II diabetes mellitus presented to the ENT clinic complaining of diplopia of 4 months duration, headache, nausea and vomiting. Ophthalmic evaluation determined diplopia for six cranial nerve palsy. Endocrinologist evaluation was normal. A magnetic resonance imaging (MRI) was performed revealing an aggressive, expansive and destructive solid mass on the infrasellar region with involvement of sphenoidal sinus and retroclival extension with high enhancement and central hypointense areas following intravenous contrast administration suggestive of calcified deposits. In addition, an expansive and destructive process was observed in cervical spine at C2 level (Fig. 1). Computed tomography (CT) of head, neck and chest was performed, showing expansive and heterodense process in skull base and cervical spine with no evidence of cervical lymphadenopathies and the thorax was normal (Fig. 2).
 Click for large image | Figure 1. Magnetic resonance imaging (MRI) of solid expansive infrasellar and cervical process with high enhancement post-intravenous injection of paramagnetic contrast. (a) T2 sequence axial view. (b) T1 fat sat and gadolinium sagittal view. (c) T1 fast sat and gadolinium axial view. |
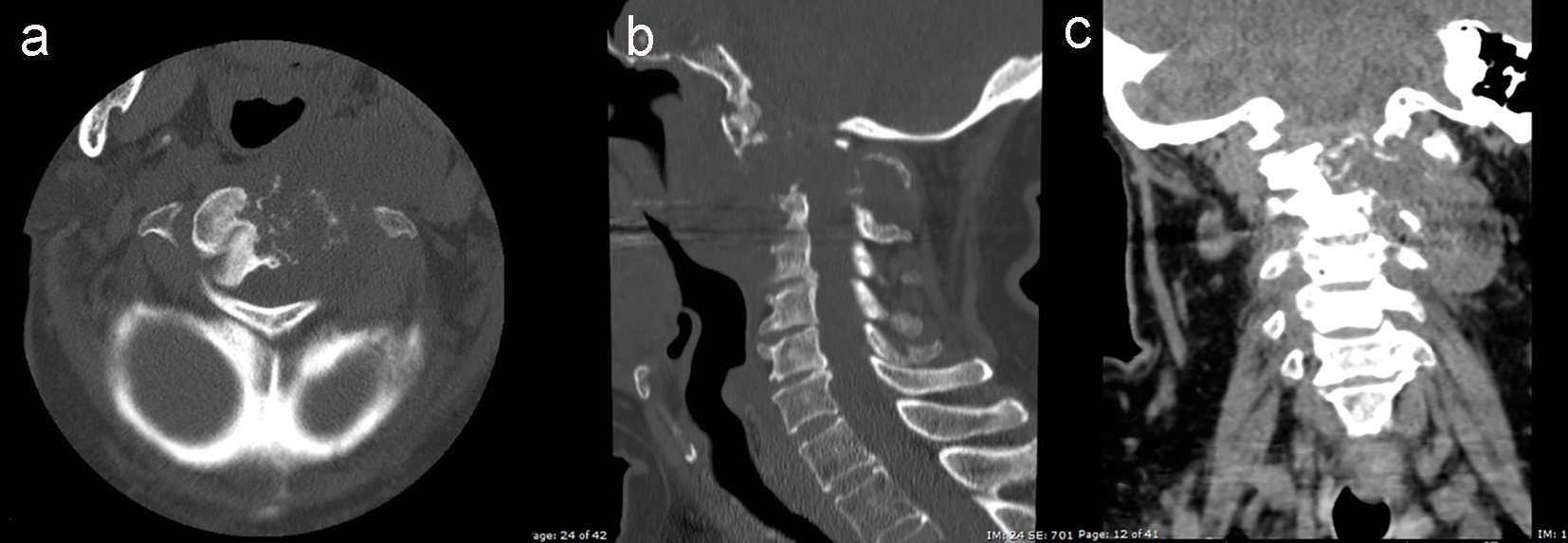 Click for large image | Figure 2. Computed tomography (CT) of expansive and destructive epidural spinal space process, centered in C2 with involvement of C1 and C3 and infiltrative compromise of neighboring left soft tissues. High enhancement after intravenous contrast injection was observed. (a) Bone window axial view. (b) Bone window sagittal view. (c) Soft tissues window and intravenous contrast, coronal view. |
A biopsy was performed under general anesthesia revealing a chondroid chordoma (CHCD). The immunohistochemistry tests were positive for protein S-100, cytokeratin AE1/AE3 and epithelial membrane antigen (EMA), and negative for glial fibrillary acidic protein (GFAP) (Fig. 3). The head and neck tumor board (HNTB) reviewed the case, where it was staged as a T4N0M0 tumor and determined out of surgical scope and recommended radiotherapy and palliative care.
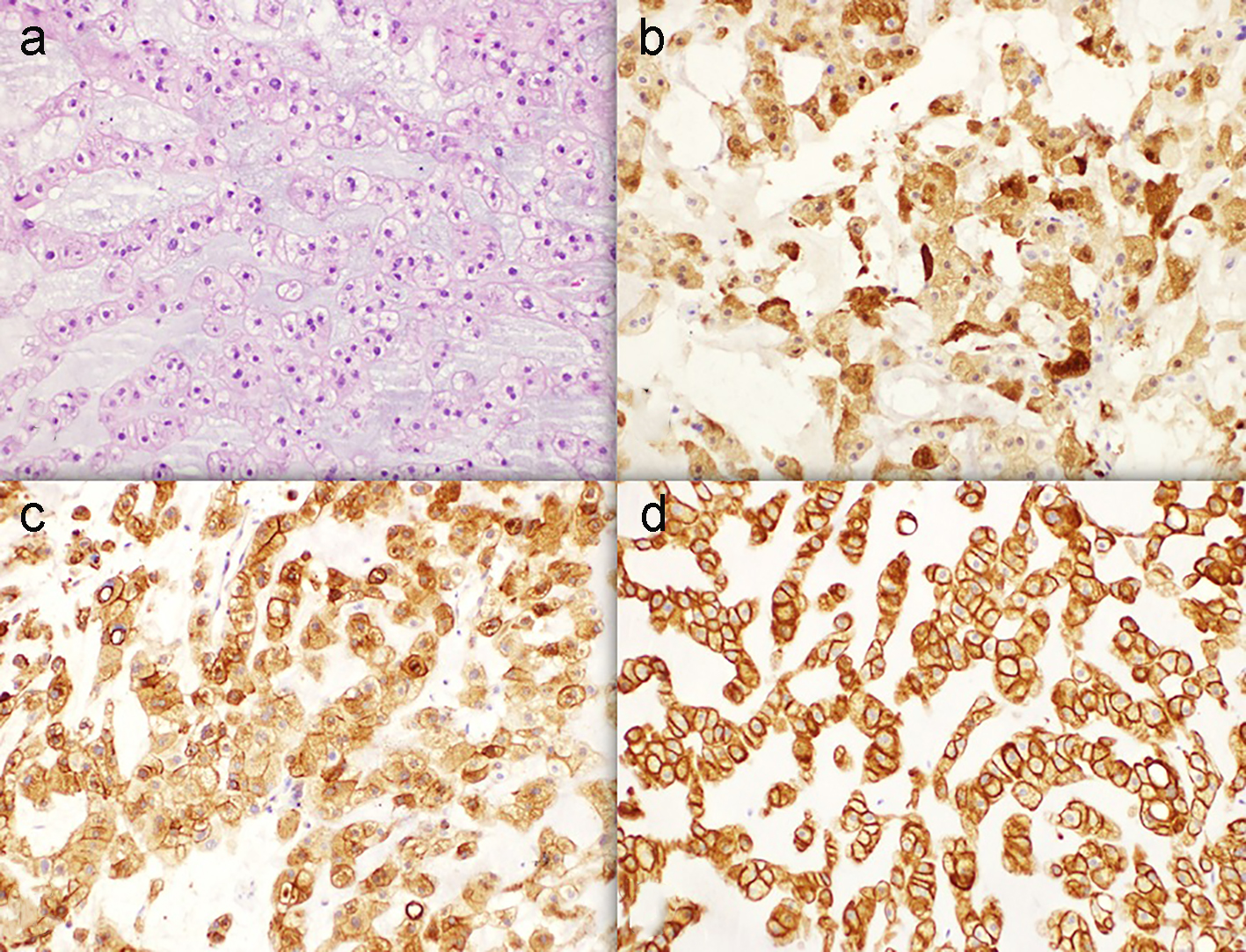 Click for large image | Figure 3. Immunohistochemistry study (× 20). (a) Hematoxylin and eosin stain. (b) Positive S-100 stain. (c) Positive EMA stain. (d) Positive KAE1/3 stain. |
Conventional palliative radiotherapy was performed with a Varian 21 iX linear accelerator, with a total dose of 36 Gy, administered in 3 Gy doses during a 2.5-week period (Fig. 4). At the end of the treatment, a neck MRI examination was performed revealing increased size of both tumors (Fig. 5). The patient was referred to the pain control and palliative care program.
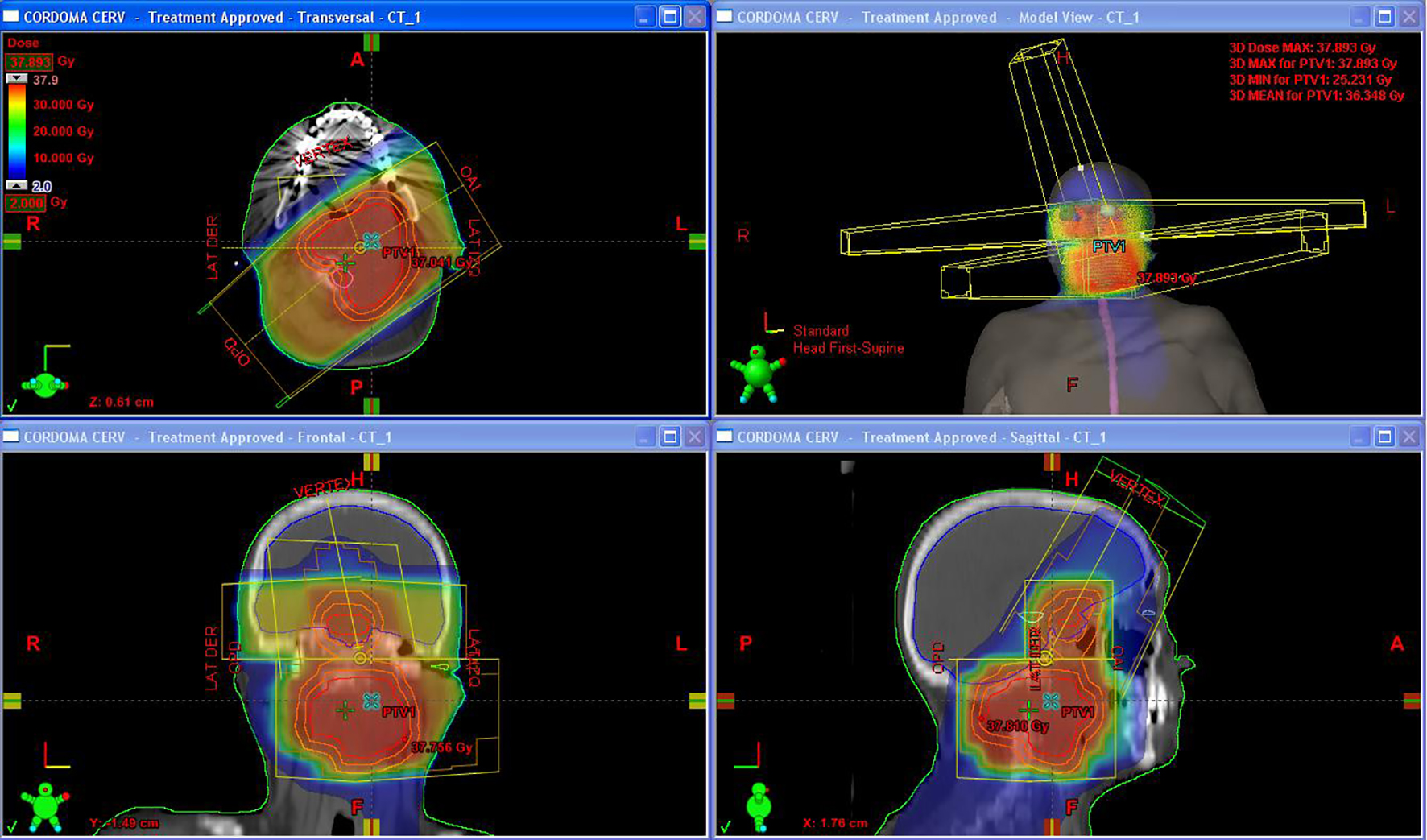 Click for large image | Figure 4. Three-dimentional conformal radiotherapy plan. |
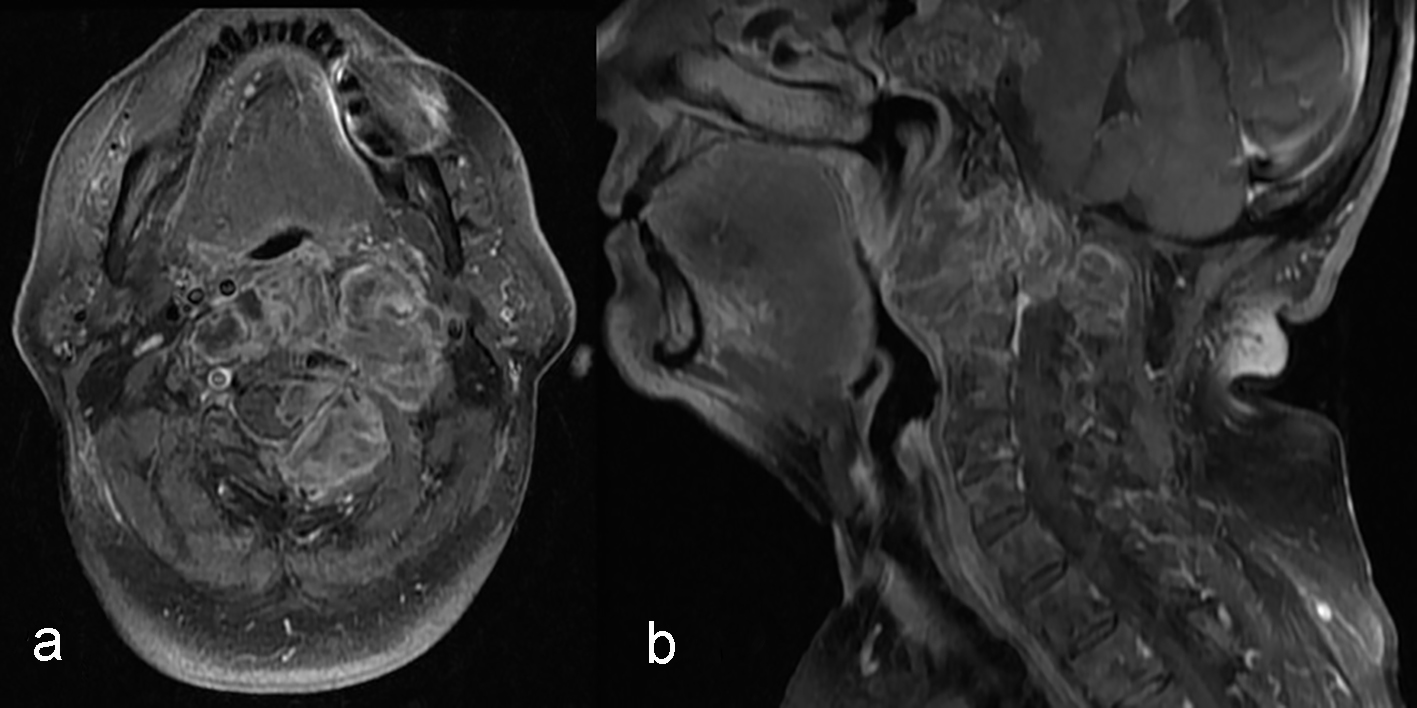 Click for large image | Figure 5. Magnetic resonance imaging (MRI) control imaging after palliative radiotherapy treatment showing growing of the expansive cervical spine process. The mass involved parapharyngeal, retropharyngeal, pharyngeal mucous space and perivertebral spaces, trespassing the middle line and involving the great vessels of the suprahyoid neck. (a) T1 fast sat and gadolinium axial view. (b) T1 fat sat and gadolinium sagittal view. |
| Discussion | ▴Top |
CDS are lesions of the middle line with low and intermediate grade of malignancy that arise from remnants of embryonic notochord in the craniovertebral axis, representing only 1-5.2% of all malignances [1]. They are most commonly found in the sacrum bone (29-60%), skull base (25-36%) and cervical spine (15-34%) [3-7].
CDS in the head and neck are uncommon, accounting for less than 0.1% of all cases of the skull base malignancies. They are characterized as slow growing in adult patients, with equal distribution between males and females, with mean ages ranging from 30 to 50 years [2].
CHCD was described first time in 1968 like a biphasic neoplasm with cartilaginous and chordal tissues in different proportions [8]. Finally, it was denominated CHCD in 1973 [5] and at present, there are less of 200 reported cases [2]. Most common locations of CHCD in the head and neck include clival region, spheno-occipital bone and sellar/parasellar region [2]. Usually the clinical findings are unspecific and depending on the location and include headache, diplopia, decrease vision, blurred vision, cranial nerve palsy (six), neck pain, nausea and vomiting [9].
Histopathologically, CHCD contains a significant amount of hyaline cartilaginous matrix in addition to the chordomatous component and are positive for protein S-100 in immunohistochemical study [10]. The histologic type is a consistent predictor of prognosis and its importance is illustrated in the American Join Committee on Cancer (AJCC) staging system.
Since CHCD is frequently associated with soft tissue invasion and bone destruction, CT and MRI are essential for preoperative staging and surgical planning in patients with these tumors [11]. CT scan findings include single hypodense and heterogeneous well-circumscribed mass with tumor calcifications. In late stage disease, the primary lesion may penetrate the cortical plate causing cortical expansion, destruction and extension into adjacent soft tissue. In MRI, in general, the CHCD appears hypointense on T1-weighted, hyperintense on T2-weighted images with inhomogeneous enhancement after intravenous gadolinium administration [2].
Complete resection is the most effective primary treatment and may include soft and hard tissues with 82% survival rate, 22.6-60.3% of morbidity and 2-7.8% of mortality [9, 12-16]. The most common complications are neurologic deficits (13.9%), cerebrospinal fluid fistula and meningitis [12]. Positive margins and residual tumor is a powerful predictor of local recurrence [1]. Neck dissection is not indicated due to the low incidence of cervical node metastasis [1]. Adjuvant radiation therapy, with doses of 65 - 70 Gy, is recommended after surgery with positive margins and regional lymph node dissection for nodal metastases [17, 18]. Proton beam therapy is the gold standard for chordomas [19]. This improves the survival rates and decreases rate of recurrences to a 25% [1, 19, 20]. Chemotherapy is not effective [9, 20, 21].
The rate of local recurrence is about 68% [22]. The rate of local and distant metastases is around 5-29% [1]. The 5-year survival rate for CHCD ranges from 51% to 82.5%, according to the histological grade of malignancy and surgical margins, and the 5-year survival rates free of disease range from 33% to 65% [1, 9, 13-16, 21, 23-27]. Strict clinical and imaging (CT) follow-up is required.
Conclusion
CHCD is a rare entity arising from remnants of embryonic notochord in the craniovertebral axis. Imaging studies play an important role in the treatment planning, providing valuable information about the growing pattern and involvement of critical anatomic organs. The treatment of choice is surgical resection, combined with adjuvant radiation therapy in some cases.
Conflicts of Interest
The authors declare that there are no actual or potential conflicts of interest in relation to this article.
| References | ▴Top |
- Yasuda M, Bresson D, Chibbaro S, Cornelius JF, Polivka M, Feuvret L, Takayasu M, et al. Chordomas of the skull base and cervical spine: clinical outcomes associated with a multimodal surgical resection combined with proton-beam radiation in 40 patients. Neurosurg Rev. 2012;35(2):171-182; discussion 182-173.
- Tsutsumi S, Akiba C, Suzuki T, Nakanishi H, Izumi H, Yasumoto Y, Ito M. Skull base chondroid chordoma: atypical case manifesting as intratumoral hemorrhage and literature review. Clin Neuroradiol. 2014;24(4):313-320.
doi pubmed - Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12(11):1344-1350.
doi pubmed - Eriksson B, Gunterberg B, Kindblom LG. Chordoma. A clinicopathologic and prognostic study of a Swedish national series. Acta Orthop Scand. 1981;52(1):49-58.
doi pubmed - Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973;32(2):410-420.
doi - McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12(1):1-11.
doi pubmed - Mirra J, Nelson S, Della Rocca C, Mertens F. Chordoma. In: Fletcher CD, Unni K, Mertens F (eds). Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. p. 316-317.
- Falconer MA, Bailey IC, Duchen LW. Surgical treatment of chordoma and chondroma of the skull base. J Neurosurg. 1968;29(3):261-275.
doi pubmed - Colli BO, Al-Mefty O. Chordomas of the skull base: follow-up review and prognostic factors. Neurosurg Focus. 2001;10(3):E1.
doi pubmed - Shintaku M, Maeno K, Okabe H. Chondroid chordoma of the skull base: immunohistochemical and ultrastructural study of two cases with special reference to microtubules within rough-surfaced endoplasmic reticulum. Med Mol Morphol. 2010;43(4):241-245.
doi pubmed - Takenaka K, Nishimura Y, Andoh T, Sakai N, Yamada H, Shimokawa K. Delayed postcontrast CT and MR imaging of chondroid chordoma - case report. Neurol Med Chir (Tokyo). 1992;32(1):28-31.
doi - Carpentier A, Blanquet A, George B. Suboccipital and cervical chordomas: radical resection with vertebral artery control. Neurosurg Focus. 2001;10(3):E4.
doi pubmed - Crockard HA, Steel T, Plowman N, Singh A, Crossman J, Revesz T, Holton JL, et al. A multidisciplinary team approach to skull base chordomas. J Neurosurg. 2001;95(2):175-183.
doi pubmed - Forsyth PA, Cascino TL, Shaw EG, Scheithauer BW, O'Fallon JR, Dozier JC, Piepgras DG. Intracranial chordomas: a clinicopathological and prognostic study of 51 cases. J Neurosurg. 1993;78(5):741-747.
doi pubmed - Gay E, Sekhar LN, Rubinstein E, Wright DC, Sen C, Janecka IP, Snyderman CH. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery. 1995;36(5):887-896; discussion 896-887.
- Yoneoka Y, Tsumanuma I, Fukuda M, Tamura T, Morii K, Tanaka R, Fujii Y. Cranial base chordoma - long term outcome and review of the literature. Acta Neurochir (Wien). 2008;150(8):773-778; discussion 778.
doi pubmed - Pearlman AW, Friedman M. Radical radiation therapy of chordoma. Am J Roentgenol Radium Ther Nucl Med. 1970;108(2):332-341.
doi pubmed - Rich TA, Schiller A, Suit HD, Mankin HJ. Clinical and pathologic review of 48 cases of chordoma. Cancer. 1985;56(1):182-187.
doi - Suit HD, Goitein M, Munzenrider J, Verhey L, Davis KR, Koehler A, Linggood R, et al. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J Neurosurg. 1982;56(3):377-385.
doi pubmed - Noel G, Feuvret L, Calugaru V, Dhermain F, Mammar H, Haie-Meder C, Ponvert D, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44(7):700-708.
doi pubmed - Almefty K, Pravdenkova S, Colli BO, Al-Mefty O, Gokden M. Chordoma and chondrosarcoma: similar, but quite different, skull base tumors. Cancer. 2007;110(11):2457-2467.
doi pubmed - Jian BJ, Bloch OG, Yang I, Han SJ, Aranda D, Tihan T, Parsa AT. Adjuvant radiation therapy and chondroid chordoma subtype are associated with a lower tumor recurrence rate of cranial chordoma. J Neurooncol. 2010;98(1):101-108.
doi pubmed - Colli B, Al-Mefty O. Chordomas of the craniocervical junction: follow-up review and prognostic factors. J Neurosurg. 2001;95(6):933-943.
doi pubmed - Benk V, Liebsch NJ, Munzenrider JE, Efird J, McManus P, Suit H. Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys. 1995;31(3):577-581.
doi - Hug EB. Review of skull base chordomas: prognostic factors and long-term results of proton-beam radiotherapy. Neurosurg Focus. 2001;10(3):E11.
doi pubmed - Igaki H, Tokuuye K, Okumura T, Sugahara S, Kagei K, Hata M, Ohara K, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. 2004;60(4):1120-1126.
doi pubmed - Samii A, Gerganov VM, Herold C, Hayashi N, Naka T, Mirzayan MJ, Ostertag H, et al. Chordomas of the skull base: surgical management and outcome. J Neurosurg. 2007;107(2):319-324.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


