| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 10, Number 8, August 2019, pages 241-245
Characteristics of Diffuse Leptomeningeal Glioneuronal Tumor With First-Episode Headache and Rapid Blindness Misdiagnosed as Viral Meningoencephalitis
Yi Baoa, c, Jing Xiaoa, Zhuru Chengb, Yan Qina, Dianyuzi Xiea, Huihui Wua, Guangjian Liua
aDepartment of Neurology, Taihe Hospital Affiliated to Hubei University of Medicine, Shiyan City, Hubei, China
bAffiliated Hospital of Guilin Medical University, Guilin, Guangxi, China
cCorresponding Author: Yi Bao, Department of Neurology, Taihe Hospital Affiliated to Hubei University of Medicine, Shiyan City, Hubei, China
Manuscript submitted July 22, 2019, accepted August 8, 2019
Short title: DLGNT Misdiagnosed as Viral Meningoencephalitis
doi: https://doi.org/10.14740/jmc3347
| Abstract | ▴Top |
Diffuse leptomeningeal glioneuronal tumor has a high degree of malignancy and high mortality. The purpose of this paper is to describe the characteristics of atypical diffuse leptomeningeal glioneuronal tumor and analyze the causes of misdiagnosis as viral meningoencephalitis. An adolescent female patient presented with headache, nausea, vomiting, sharp vision loss and cognitive dysfunction. After poor therapeutic effect of standard antiviral treatment, further inspection found that malignant cells were detected by cerebrospinal fluid (CSF) cytology; and enhanced magnetic resonance imaging (MRI) showed extensive enhancement of the leptomeningeal. In conclusions, when patient with unexplained high intracranial pressure, it is necessary to be alert to the diagnosis of diffuse leptomeningeal glioneuronal tumor. Multiple examinations of fresh CSF are helpful to increase the positive detection rate of tumor cells. Early diagnosis and active treatment are conducive to improving survival rate.
Keywords: Leptomeningeal glioneuronal tumor; Viral meningoencephalitis; CSF; Cytology
| Introduction | ▴Top |
Diffuse leptomeningeal glioneuronal tumor (DLGNT) is a special type of central nervous system disease in which malignant tumor cells metastasize and infiltrate (diffusely or focally) the pia mater and subarachnoid space. Primary lesions are mostly from lung cancer, breast cancer, etc. The most common initial symptoms are headache, nausea, vomiting and meningeal irritation symptoms, which often accompanied by visual hallucinations and decline in cognitive function. The disease has a high mortality and disability rate, poor prognosis and a variety of clinical manifestations that are not specific, so it is easily misdiagnosed. The previous antemortem diagnosis rate is extremely low. With the improvement of clinical knowledge and examination level, more and more patients can be clinically discovered. Cerebrospinal fluid (CSF) cytology is the gold standard for the diagnosis; and magnetic resonance imaging (MRI) has important clinical value for early diagnosis of meningeal cancer [1-3].
Viral meningoencephalitis is a common infectious disease of the central nervous system with clinical manifestations of fever, headache, consciousness and mental behavior changes, often accompanied by epilepsy and focal nerve defects. Clinically, the severity of the disease varies. Mild cases can be self-limited; severe cases can lead to serious sequelae and even death. Early clinical manifestations, CSF, and brain imaging changes are not typical. This leads to the disease with high misdiagnosis rate, high disability rate and high mortality rate [4-6].
In view of the similar clinical manifestations of DLGNT and viral meningoencephalitis, both have atypical conditions; and the diagnosis usually depends on CSF and MRI. Due to the low incidence and poor prognosis of DLGNT, clinicians often regard tumor cells as an exclusive diagnosis before the detection of tumor cells, and it is easy to be misdiagnosed as viral meningitis at the first visit.
This article reports the diagnosis and treatment of an adolescent female patient with headache, nausea, vomiting, sharp vision loss and cognitive dysfunction. In the early stage of onset, since no cancer cells were found in the CSF, and the MRI features were similar to those of viral meningoencephalitis, the patient was diagnosed as viral meningoencephalitis. After standard antiviral treatment, the patient did not get better. After repeated CSF examinations, heteromorphic cells were found, and the patient was finally diagnosed as DLGNT. The identification of the disease with optic neuromyelitis, venous sinus thrombosis, tuberculous meningitis, etc., is described in detail in other articles [7, 8].
| Case Report | ▴Top |
A 16-year-old girl was admitted to the hospital because of headache and vomiting for 20 days, which aggravated with decreased vision for 3 days. Twenty days ago, she had headache, nausea and vomiting, no fever, no obvious cause, and no improvement after infusion therapy (cephalosporins) at the local clinic. The patient had frequent vomiting and poor appetite. She went to the gastroenterology department for treatment, but her symptoms still aggravated after symptomatic treatment. In the past 3 days, she had blurred vision and progressive vision loss, accompanied by unconsciousness, so she was transferred to our hospital for emergency treatment. She had always been in good health, no history of hepatitis or tuberculosis, no history of trauma surgery, no history of food or drug allergy, no history of exposure to toxic substances, full-term delivery, and no family history.
Physical examination results
Her vitals on admission were: temperature 36.8 °C, pulse 72 beats/min, respiratory rate 19/min, blood pressure 106/71 mm Hg. She was wheeled into the ward, physical examination cooperation. There was no yellow staining or bleeding spots on the skin mucosa, no swelling of superficial lymph nodes, and no positive signs on cardiopulmonary and abdominal examination. Neurological examination revealed that somnolence; poor memory; the right side of the eye fissure was small; the pupils of both sides were unequal with the diameter of the right side 5mm, insensitive to light reflection, and the diameter of the left side 4mm, slow to light reflection; both eyes move freely in all directions, no nystagmus, and no blurred vision. Bilateral frontal stria and nasolabial groove were symmetrical; the tongue was in the middle. She could speak clearly; and pharyngeal reflex existed. Muscle strength and muscle tension of the limbs were normal; bilateral tendon reflex was negative; bilateral deep sensation, shallow sensation and ataxia movement examination were normal; and pathological signs were negative; neck stiffness (five transverse fingers), bilateral Kernig’s sign and Brudzinski’s sign were positive.
General inspection results
The head computed tomography (CT) and electroencephalogram (EEG) were normal. Abdominal CT showed gallstones, small kidney stones. Blood routine revealed: white blood cell (WBC) 14.4 × 109/L, neutrophil granulocytes (NE) 12.53 × 109/L, lymphocytes (LY) 1.08 × 109/L, red blood cell (RBC) 4.79 × 1012/L, hemoglobin (Hb) 145 g/L, hematocrit (HCT) 40.7, platelets (PLT) 280 × 109/L; erythrocyte sedimentation rate (ESR) 4 mm/h; myocardial enzyme spectrum, liver and kidney function, electrolyte and coagulation function were normal. Brain MRI plain scan: bilateral hippocampus, right thalamic abnormal signal, high possibility of virus infection, the supratentorial ventricle slightly enlarged. Lumbar puncture examination results: CSF pressure was greater than 400 mm H2O (1 mm H2O = 0.0098 kPa); CSF routine: light yellow, sight turbidity, no clot, Pan’s test (Pandy) positive, total cells number 20 × 109/L, WBC count 13 × 109/L; Cryptococcus neoformans polysaccharide antigen (LA) negative (-), no acid-fast bacilli, no cryptococcus; CSF biochemistry: protein 4.97 g/L (high), Cl 105.2 mmol/L (low), glucose 4.98 mmol/L (high), lactate dehydrogenase (LDH) 34 U/L, high-sensitivity C-reactive protein (hsCRP) 0.37 mg/L, adenosine deaminase (ADA) 0.41 U/L. Viral antibody examinations showed: cytomegalovirus (CMV)-IgG positive (+), Rub-IgG positive (+), herpes simplex virus (HSV)I-IgG positive (+), the others were negative. Serum tumor marker results showed no abnormalities.
Treatment and disease progression
After admission, she was treated with antiviral therapy (ganciclovir), nerve nutrition, dehydration, decreasing intracranial pressure therapy (mannitol, glycerol fructose, albumin, and furosemide), electrolyte supplementation and nutritional support. On the second day of admission, the patient’s vision dropped to a sense of no light, and she could not distinguish day from night. For the patient’s vision loss, we consulted the ophthalmology. Examination of the ophthalmoscope found that the edge of the nipple was unclear, the physiological depression disappeared, the retinal vein was filled with distortion, and the retinal edema around the nipple was turbid. According to the condition, the cranial hypertension syndrome was considered; and the pupils were unequal, considering the formation of cerebral edema and cerebral palsy. It was needed to relieve the craniocerebral pressure urgently, but the patient’s intracranial pressure was extremely high, direct lumbar puncture and drainage of CSF would have the risk of developing cerebral hernia, which is life-threatening. After we consulted the neurosurgery and considering the cause of high blood pressure syndrome, emergency Ommaya sac implantation was done, with drainage of light yellow CSF about 300 mL per day. The patient’s cranial hypertension was relieved, and the patient’s visual acuity gradually recovered after treatment.
Differential diagnosis
After standard antiviral treatment for half a month, there was no decrease in CSF drainage. The pathogenic test results of the CSF sent to Huada Gene Company showed that no related gene fragments were found in viruses, bacteria, fungi, tuberculosis or parasites. In order to find out the cause, fresh CSF was repeatedly sent for examination, and enhanced magnetic resonance examination was performed. The cytology of CSF showed heterogenous cells with prominent malignant features. The cells were scattered and varied in size, the cell membrane was malformed, the ratio of nucleus to cytoplasm was significantly unbalanced, the nucleoli were active, and the nucleoli accounted for the majority of chromatin. Enhanced magnetic resonance imaging showed extensive enhancement of the leptomeningeal, especially in the brainstem and tentorium cerebellum, as shown in Figure 1.
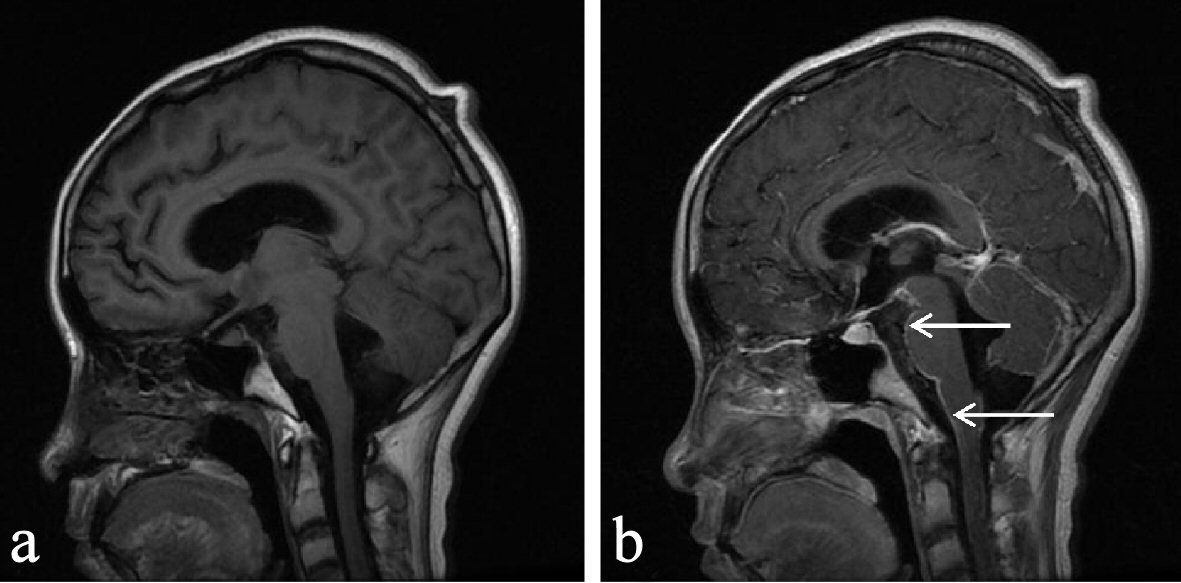 Click for large image | Figure 1. (a) MRI plain scan: no obvious abnormality was observed. (b) Mass injection of Gd-DTPA enhanced scan: small patchy abnormal enhancement lesion can be seen in the sagittal parietal occipital lobe, with irregular shape and unclear boundary; multiple linear and nodular abnormal lesion with enhancement can be seen in the intracranial perichondrium (arrow); right thalamus neoplastic lesions and extensive meningeal metastasis are possible. |
Diagnosis and treatment
CSF cytology and enhanced MRI results confirmed the diagnosis of meningeal carcinoma. According to the classification of central nervous system tumors of the WHO in 2016 [9, 10], the results were consistent with DLGNT with high malignancy. After we consulted the oncology department, chemotherapy was suggested, but the patient’s condition was gradually progressing, spinal cord metastasis occurred as follows: decreased lower extremity sensation, decreased muscle strength, and urinary retention. Finally, the patient’s family gave up further treatment.
| Discussion | ▴Top |
In 1870, Eberth first discovered meningeal cancerous lesions from autopsy of lung cancer cases [11]. In 1912, Beer Man first named the disease as meningeal cancer [12]. It has been widely recognized as a rare disease. With the progress of imaging research and the prolonged survival of cancer patients, more and more meningeal cancer has been reported in the literature [1-3]. According to statistics, the incidence rate accounts for 5-8% of cancer patients, and the clinical manifestations have no obvious specificity. Most of them are headache, vomiting, etc., and the meningeal irritation sign is positive, also known as cancerous meningitis. Some patients may have epilepsy, disturbance of consciousness and mental retardation. Some patients may have hemiplegia, visual and auditory disorders. When the spinal cord and nerve roots are involved, the corresponding regional motor dysfunction may occur; and in severe cases, bladder rectal dysfunction may occur [13, 14].
The current diagnostic criteria for meningeal carcinoma are as follows: 1) Having a history of tumor; 2) New signs of meninges stimulation; 3) Cancer cells found in CSF cytology; 4) Typical MRI findings. Meningeal carcinoma without parenchymal metastasis and no significant contrast between the diseased tissue and the adjacent CSF make MRI plain scan difficult to detect meningeal abnormalities. Enhanced MRI can show pathological changes, so it has become one of the preferred imaging methods for the diagnosis of meningeal cancer. The main features are: 1) The pia mater, subarachnoid diffuse or nodular enhancement; 2) Diffuse or nodular enhancement of the ependymal membrane; 3) Irregular thickening and strengthening of the canopy; 4) Traffic hydrocephalus. It can detect not only the type of invasion of the meninges, but also whether there is a lesion in the brain parenchyma, and sometimes find a strengthening of the spinal nerve roots; and still it can find spinal nerve root aggrandizement sometimes. According to previous literature reports [2, 3], meningeal enhancement is caused by the blockage of meningeal venules by tumor emboli, which leads to the high expansion of blood vessels in the inner meninges and the increase of vascular permeability. This patient has typical meningeal enhancement, which is manifested as right thalamus lesion and lamellar meningeal enhancement. However, CSF cytology is still the gold standard for the diagnosis of meningeal carcinoma [15, 16].
In clinical practice, DLGNT is not common. Under normal circumstances, MRI examination find that the frontotemporal lobe and basal ganglia (thalamus) and other parts of the lesions; lesions can be large, “fusion” changes, involving gray matter, edema; and white matter can also be affected [17, 18]. CSF examination shows that the pressure is extremely high, WBCs are slightly increased, protein is high, sugar and chloride are normal, and most of them are diagnosed as viral meningoencephalitis, which is an important reason for misdiagnosis caused by DLGNT. However, viral meningoencephalitis is a self-limiting disease with a course of several weeks to several months. After standard antiviral therapy, it can return to normal. The currently used drug is ganciclovir, a broad-spectrum anti-DNA virus agent that exerts antiviral effects by directly penetrating viral DNA and competitively inhibiting the binding of GTP to viral DNA. This case combined with the first diagnosis of symptoms, signs, viral antibodies, CSF and MRI plain scan results, was misdiagnosed as viral meningoencephalitis; but standard antiviral treatment was invalid. Finally, a DLGNT was diagnosed by repeated CSF cytology.
The main characteristics of the patient were: severe nausea and vomiting, rapid vision loss, and changes in conscious level. CSF was characterized by ultra-high intracranial pressure, extremely high protein, excessive sugar (normal blood sugar), slightly lower chloride, and no primary lesions were found outside the skull. The specific analysis is as follows: first of all, where is the primary lesion? According to literature reports, most tumor cells are caused by tumor metastasis from other sites, and the primary lesions are often from lung cancer, breast cancer, etc. The routes for tumor cells to reach the meninges include: 1) Blood type dissemination through Baston’s venous plexus or arteries; 2) Direct spread from adjacent intracranial primary tumor lesions or brain metastatic tumor lesions; 3) Tumors from the whole body migrate to the center along the peripheral nerve or vascular space. After admission, no tumors were found in the patient’s neck, chest or abdomen. Only abnormal signals in the right thalamus were found on MRI plain scan of the brain. It was considered that the primary lesion spread directly to the meninges in the right thalamus and caused a series of symptoms [19-21]. Second, ultra-high intracranial pressure manifested as headache, neck pain, nausea and vomiting, rapid decline in visual acuity, positive signs of meningeal irritation, neck strength five horizontal fingers, and the CSF pressure was much greater than 400 mm H2O. Visual impairment showed no light in both eyes, and the pupils on both sides were not equal. The diameter of the right side was 5 mm, and the diameter of the left side was 4 mm. Both eyes and indirect light reflection disappeared. The cause of the patient’s intracranial hypertension might be the results of the deposition of tumor cells that blocked the CSF outflow pathway. The patient had a very high intracranial pressure and severe visual impairment, which was inconsistent with most reports [22, 23]. The third one is the significant protein cell separation. The patient’s CSF protein was extremely high, and WBCs were in normal range. These are consistent with diffuse pial glial neuronal tumor lesions, but diffuse pial glial neuronal tumors with CSF protein levels greater than 4 g/L are very rare. The reasons for this include tumor cells infiltrating the meninges, chemical stimulation of tumor metabolites, destruction of the blood-brain barrier, and increased vascular permeability, resulting in increased protein exudation.
Another characteristic of this patient was that CSF sugar exceeds the standard, even when the patient had obvious nausea, vomiting and poor appetite, the measured blood glucose was lower than the reference value, while the measured CSF sugar was higher than the normal value, or even higher than the blood glucose, which is inconsistent with the literature report [23]. Increased CSF sugar is usually seen in patients with increased blood sugar, central nervous system infection, brain injury after cranial bottom concave and III ventricle tumors and high fever, etc., which were associated with increased permeability of blood brain barrier. The reason for the increase in glucose in this patient was that the increase in blood-brain barrier permeability caused by DLGNT was related, but it was difficult to explain the fact that CSF sugar was higher than blood glucose.
DLGNT is a malignant tumor with high degree, rapid onset, short course, poor treatment effect and high mortality. Current treatment methods include: surgery, radiotherapy, systemic chemotherapy, intrathecal chemotherapy, targeted therapy, immunotherapy and support therapy. The operation has two main purposes: 1) Patients are given intraventricular chemotherapy by burying Ommaya sac under the scalp; 2) Ventriculoperitoneal shunt is recommended for patients with high cranial pressure. Targeted therapies mainly include EGFR tyrosine kinase inhibitors, ALK inhibitors, HER2 monoclonal antibodies, and vascular endothelial growth factor monoclonal antibodies [24-26].
The shortcomings of this paper are: 1) Lack of experience in the diagnosis and treatment of DLGNT, leading to early misdiagnosis as viral meningoencephalitis; 2) No meningeal biopsy, no accurate tumor typing; 3) There is no positron emission tomography/computed tomography (PET/CT) examination, and the primary tumors in other parts of the body cannot be completely excluded; 4) There is no genetic test, and no precise treatment under the guidance of the gene is provided; 5) Olig-2, S-100, Syn, BRAF fusion and chromosome 1p deletion detection were not performed [27].
In conclusion, DLGNT have high malignancy, high mortality, diverse clinical manifestations; and early diagnosis is difficult. It is difficult to make the right diagnosis in most patients before antemortem. Survival depends largely on early diagnosis and early active treatment; early diagnosis is especially important. At present, enhanced MRI can aid diagnosis, CSF cytology is the gold standard for diagnosis, but most patients with diffuse pial glial neuron tumor disease lack typical clinical symptoms and signs; and it is difficult for first-time doctors to think of DLGNT without enhanced MRI and CSF cytology results. Through this case, we summarize the following experiences: 1) When patients with unexplained high intracranial pressure, and there are difficulties in the diagnosis; it is needed to be alert to DLGNT; 2) When MRI plain scan does not find lesions, but the condition cannot be explained, it is necessary to perform the enhanced MRI in time; 3) CSF cytology needs to be sent repeatedly to avoid false negatives caused by time delay between taking liquid and examination; 4) For patients with intracranial hypertension, Ommaya sac should be placed in time to relieve pressure and save vision.
Acknowledgments
We are grateful to the medical staff members who have treated this patient carefully.
Financial Disclosure
This is a clinical observation article without any funding.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The patient’s informed consent was obtained.
Author Contributions
YB, JX and ZRC summed up and wrote the article; ZRC and YQ wrote the article; DYZX, HHW and GJL collected the case data.
| References | ▴Top |
- Gardiman MP, Fassan M, Orvieto E, D'Avella D, Denaro L, Calderone M, Severino M, et al. Diffuse leptomeningeal glioneuronal tumors: a new entity? Brain Pathol. 2010;20(2):361-366.
doi pubmed - Cho HJ, Myung JK, Kim H, Park CK, Kim SK, Chung CK, Choi SH, et al. Primary diffuse leptomeningeal glioneuronal tumors. Brain Tumor Pathol. 2015;32(1):49-55.
doi pubmed - Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, Laurence V, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21(11):2183-2187.
doi pubmed - Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, Kennedy PG. Viral meningoencephalitis: a review of diagnostic methods and guidelines for management. Eur J Neurol. 2010;17(8):999-e957.
doi pubmed - Tyler KL. Acute Viral Encephalitis. N Engl J Med. 2018;379(6):557-566.
doi pubmed - Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology. 2015;84(4):359-366.
doi pubmed - Jing Xiao, Lei Gao, Miao Zhang, et al. Clinical features of diffuse leptomeningeal glioneuronal tumor with rapid blindness misdiagnosed as NMOSD and literature review. SN Comprehensive Clinical Medicine. 2019;1(6):434-441.
doi - Yi Bao, Yajie Hu, Yayong Ding, et al. Clinical features of DLGNT with the first symptom of headache and decreased vision misdiagnosed as venous sinus thrombosis. Journal of Neurology Research. 2019;9(3):41-47.
doi - Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, et al. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820.
doi pubmed - Diamandis P, Aldape K. World Health Organization 2016 Classification of Central Nervous System Tumors. Neurol Clin. 2018;36(3):439-447.
doi pubmed - Fischer-Williams M, Bosanquet FD, Daniel PM. Carcinomatosis of the meninges; a report of three cases. Brain. 1955;78(1):42-58.
doi pubmed - Beerman WF. Meningeal carcinomatosis. Journal of the American Medical Association. 1912;58(19):1437-1439.
doi - Little JR, Dale AJ, Okazaki H. Meningeal carcinomatosis. Clinical manifestations. Arch Neurol. 1974;30(2):138-143.
doi pubmed - de Azevedo CR, Cruz MR, Chinen LT, Peres SV, Peterlevitz MA, de Azevedo Pereira AE, Fanelli MF, et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011;104(2):565-572.
doi pubmed - Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38(1):51-57.
doi pubmed - Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25(2):103-119.
doi pubmed - Watanabe M, Tanaka R, Takeda N. Correlation of MRI and clinical features in meningeal carcinomatosis. Neuroradiology. 1993;35(7):512-515.
doi pubmed - Lin X, Fleisher M, Rosenblum M, Lin O, Boire A, Briggs S, Bensman Y, et al. Cerebrospinal fluid circulating tumor cells: a novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2017;19(9):1248-1254.
doi pubmed - Kuruppu D, Bhere D, Farrar CT, Shah K, Brownell AL, Tanabe KK. A model of breast cancer meningeal metastases: characterization with in vivo molecular imaging. Cancer Gene Ther. 2019;26(5-6):145-156.
doi pubmed - Garzia L, Kijima N, Morrissy AS, De Antonellis P, Guerreiro-Stucklin A, Holgado BL, Wu X, et al. A Hematogenous Route for Medulloblastoma Leptomeningeal Metastases. Cell. 2018;172(5):1050-1062 e1014.
- Yeamans CL, Gutierrez-Quintana R, Haley A, Lamm CG. Magnetic resonance imaging and clinical findings associated with choroid plexus spinal cord "Drop" metastases. J Am Anim Hosp Assoc. 2017;53(5):265-269.
doi pubmed - Nusbaum DM, Wu SM, Frankfort BJ. Elevated intracranial pressure causes optic nerve and retinal ganglion cell degeneration in mice. Exp Eye Res. 2015;136:38-44.
doi pubmed - Ducros A, Biousse V. Headache arising from idiopathic changes in CSF pressure. Lancet Neurol. 2015;14(6):655-668.
doi - Blaszczak W, Barczak W, Wegner A, Golusinski W, Suchorska WM. Clinical value of monoclonal antibodies and tyrosine kinase inhibitors in the treatment of head and neck squamous cell carcinoma. Med Oncol. 2017;34(4):60.
doi pubmed - Yu HA, Tian SK, Drilon AE, Borsu L, Riely GJ, Arcila ME, Ladanyi M. Acquired resistance of EGFR-mutant lung cancer to a T790M-Specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 2015;1(7):982-984.
doi pubmed - Lavaud P, Rousseau B, Ajgal Z, Arrondeau J, Huillard O, Alexandre J, Hulin A, et al. Bi-weekly very-high-dose lapatinib: an easy-to-use active option in HER-2-positive breast cancer patients with meningeal carcinomatosis. Breast Cancer Res Treat. 2016;157(1):191-192.
doi pubmed - Rout P, Chand A, Nandeesh B, et al. Clinicopathological study of recently added glioneuronal tumors. Clinical Cancer Investigation Journal. 2017;6(2):128-128.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


