| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 12, Number 2, February 2021, pages 65-70
Aerococcus urinae: A Rare Cause of Endocarditis Presenting With Acute Stroke
Yasir Ahmeda, d, Nikky Bardiaa, Caleb Judgea, Sajjad Ahmadb, Christopher Malozzib, Eduardo Calderonc
aDepartment of Internal Medicine, University of South Alabama, Mobile, AL, USA
bDepartment of Cardiology, University of South Alabama, Mobile, AL, USA
cDepartment of Infectious Diseases, University of South Alabama, Mobile, AL, USA
dCorresponding Author: Yasir Ahmed, Department of Internal Medicine, University of South Alabama, 2451 USA Medical Center Drive, 4th Floor Mastin Building, Mobile, AL 36617, USA
Manuscript submitted October 20, 2020, accepted November 25, 2020, published online December 30, 2020
Short title: Aerococcus urinae
doi: https://doi.org/10.14740/jmc3612
| Abstract | ▴Top |
Aerococcus urinae is a rare organism infrequently isolated from cultures. Mostly known to cause urinary tract infection, it can cause bacteremia leading to severe urosepsis and infective endocarditis. Embolization is frequently reported with Aerococcus urinae endocarditis (AUE); hence, the presentation is highly variable. Sequelae such as various central nervous system manifestations, sepsis, valvular regurgitation with heart failure and even coronary artery involvement have been reported. We report a case of a 58-year-old man with AUE of the aortic valve, severe aortic regurgitation and embolic stroke as a result of embolization from AUE and ultimately required aortic valve replacement. Our case highlights this rare cause of endocarditis and offers insight into the variability of patient presentation and risk factors to consider.
Keywords: Aerococcus urinae; Aerococcus urinae endocarditis; Infective endocarditis
| Introduction | ▴Top |
Aerococcus urinae (AU) is an alpha-hemolytic, coagulase-negative, gram-positive coccus. These features are similar to streptococci and enterococci species and it also resembles staphylococci as it forms in clusters [1]. This explains why it has historically been misdiagnosed. As isolation techniques improve, aerococcus is being identified more frequently. It was initially described in 1938 and was referred to as an “altered streptococci” and it was thought to be a non-pathogenic contaminant [2]. In the early 1950s, it was identified as a single species [3]. Due to advancements in identification techniques, aerococci were further divided into A. viridians and A. urinae using 16sRNA sequencing in 1992 [4]. So far seven species of aerococcus have been identified, two of which are not found in humans. The species are ubiquitous in the environment. It can be found in soil, air and in the microbiota of certain mammals. We report a case of Aerococcus urinae endocarditis (AUE) that presented as an acute thromboembolic stroke and managed with intravenous (IV) antibiotics and aortic valve replacement (AVR).
| Case Report | ▴Top |
A 58-year-old African-American man with hypertension, hyperlipidemia and polysubstance abuse presented with dysarthria and unsteady gate. His speech was incoherent, was slow to comprehend and his gait was also abnormal that was described as “wobbly” by his brother. No specific focal deficits were reported. He had recently developed urinary incontinence and was treated for a possible urinary tract infection (UTI) within the preceding 2 months. He smoked almost a pack a day for almost 45 years and was drinking a bottle of liquor every other day. On physical examination, he was vitally stable, alert and oriented but had a blunted affect. Although slow to comprehend, he was able to follow commands, had noticeable dysarthria and difficulty with balance while ambulating, but not to the point of falling. Rest of the neurologic exam including cranial nerves, sensory and motor systems was grossly intact. Cardiac exam revealed regular rate and rhythm with an II/VI diastolic murmur at the left sternal border. Physical exam also revealed phimosis.
Initial computed tomography (CT) of the head without contrast was negative for intracranial hemorrhage (ICH). However, it did reveal patchy bifrontal white matter hypodensities (Fig. 1). Neurology was consulted for stroke evaluation as the findings were deemed suspicious for cardioembolic source of stroke. Acute infarcts in the middle cerebral artery (MCA) territory and to a lesser extent the anterior cerebral artery (ACA) distributions were reported on magnetic resonance imaging (MRI) of the brain (Fig. 2). Magnetic resonance angiography (MRA) of the head and neck was normal. The initial transthoracic echocardiogram (TTE) showed 8 × 9 mm aortic valve vegetation with significant aortic regurgitation. The subsequent transesophageal echocardiogram (TEE) reported an 18 × 5 mm vegetation on the aortic valve (Fig. 3) with severe, eccentric aortic regurgitation (Fig. 4). The patient was given a diagnosis of possible endocarditis as he met one major criterion (positive TTE for vegetation) and one minor criterion (embolic phenomenon) of the Duke criteria for infective endocarditis (IE). Antibiotics were held initially as the patient did not have sign and symptoms of infection or sepsis. Daily blood cultures were obtained for the first 3 days which were preliminarily reported negative. However, the patient developed fever and tachycardia on day 5 of the hospitalization and was started on empiric vancomycin and cefazolin. The following day, blood cultures exhibited growth of gram-positive cocci and were later identified as AU (Fig. 5). An episode of transient facial droop and weakness also occurred during this time that resolved spontaneously likely representing a transient ischemia attack (TIA).
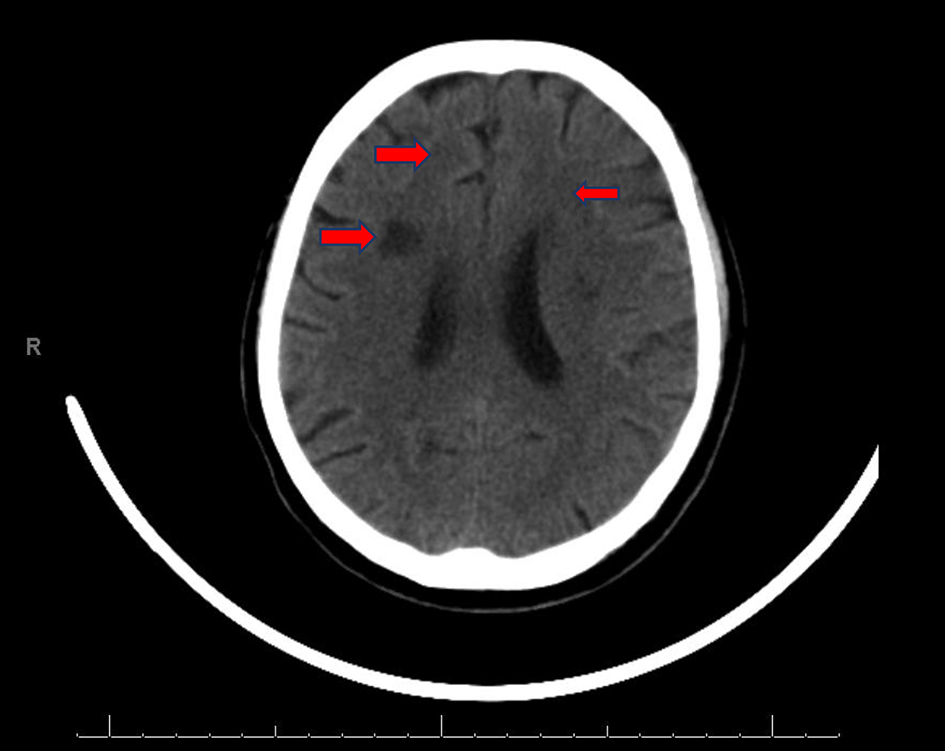 Click for large image | Figure 1. CT of head WO: patchy bifrontal white matter hypodensities (red arrows). CT: computed tomography; WO: without contrast. |
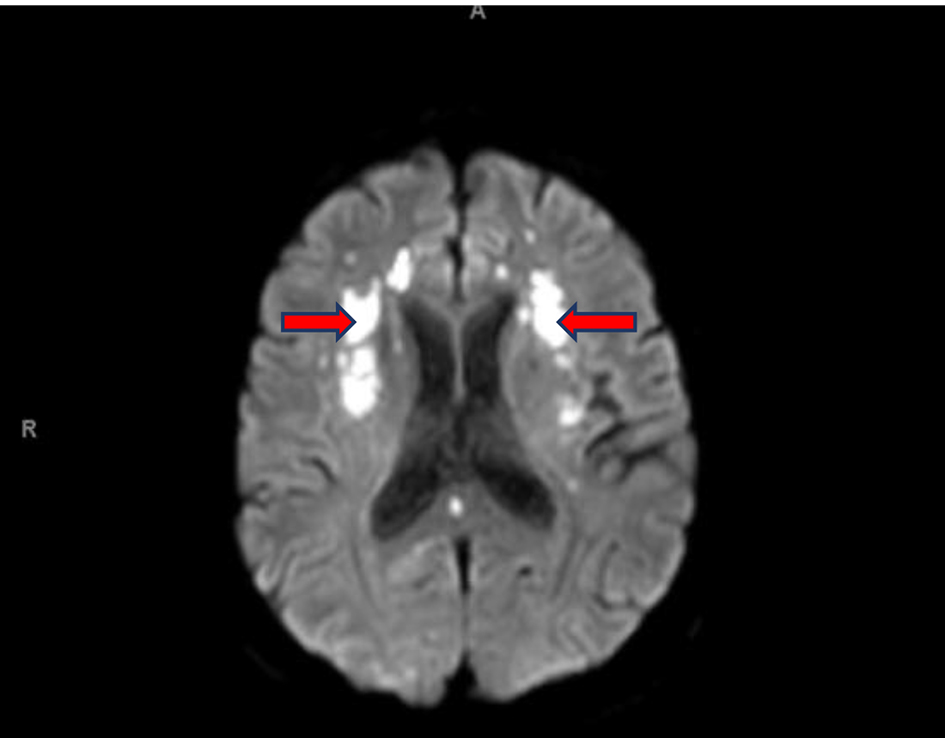 Click for large image | Figure 2. MRI of head WO: acute infarcts in the MCA distributions and ACA distributions, to a lesser extent (red arrows). MRI: magnetic resonance imaging; WO: without contrast; MCA: middle cerebral artery; ACA: anterior cerebral artery. |
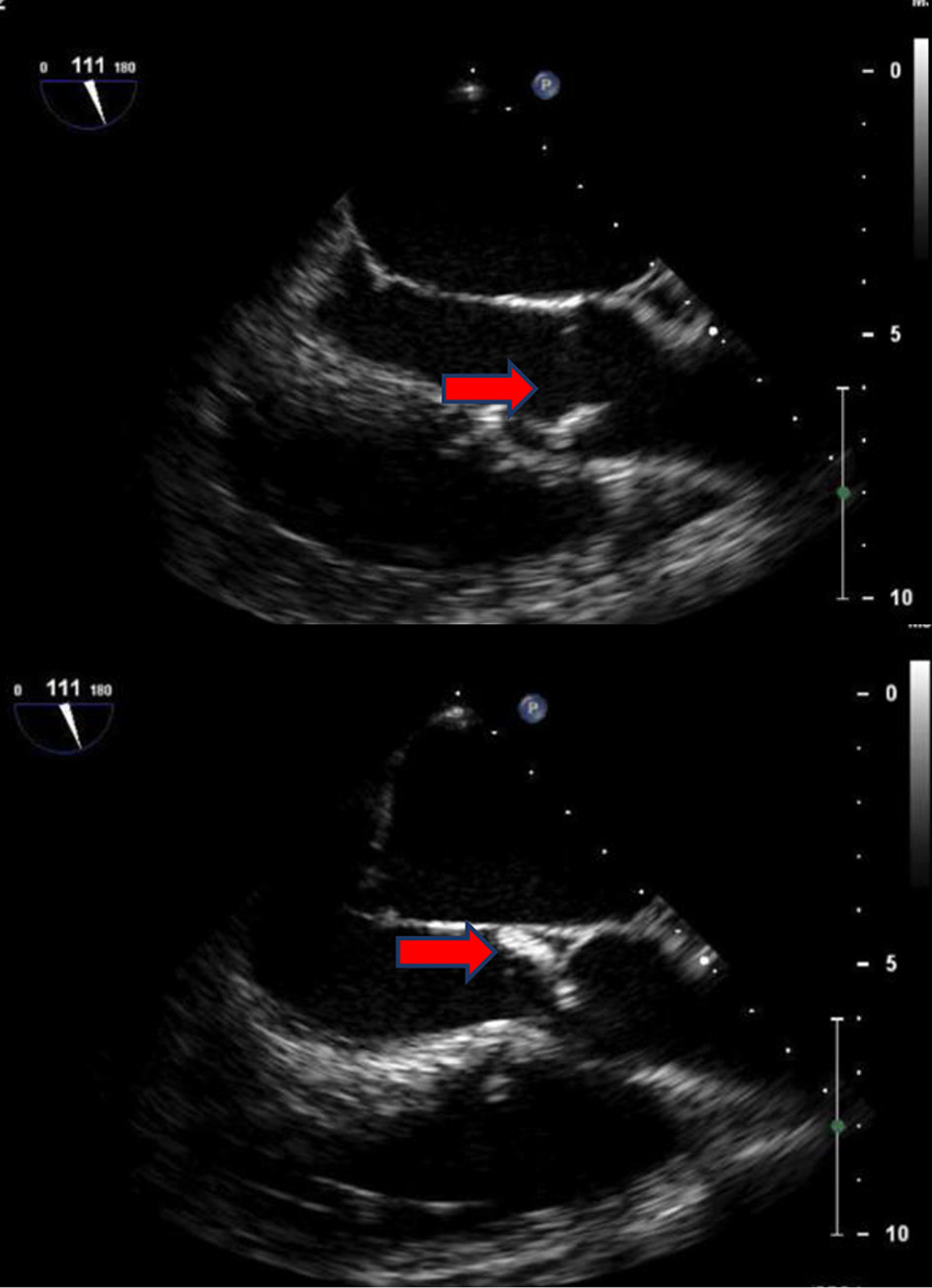 Click for large image | Figure 3. TEE: a large mobile vegetation is seen on right coronary cusp with severe aortic valve regurgitation (red arrows). TEE: transesophageal echocardiogram. |
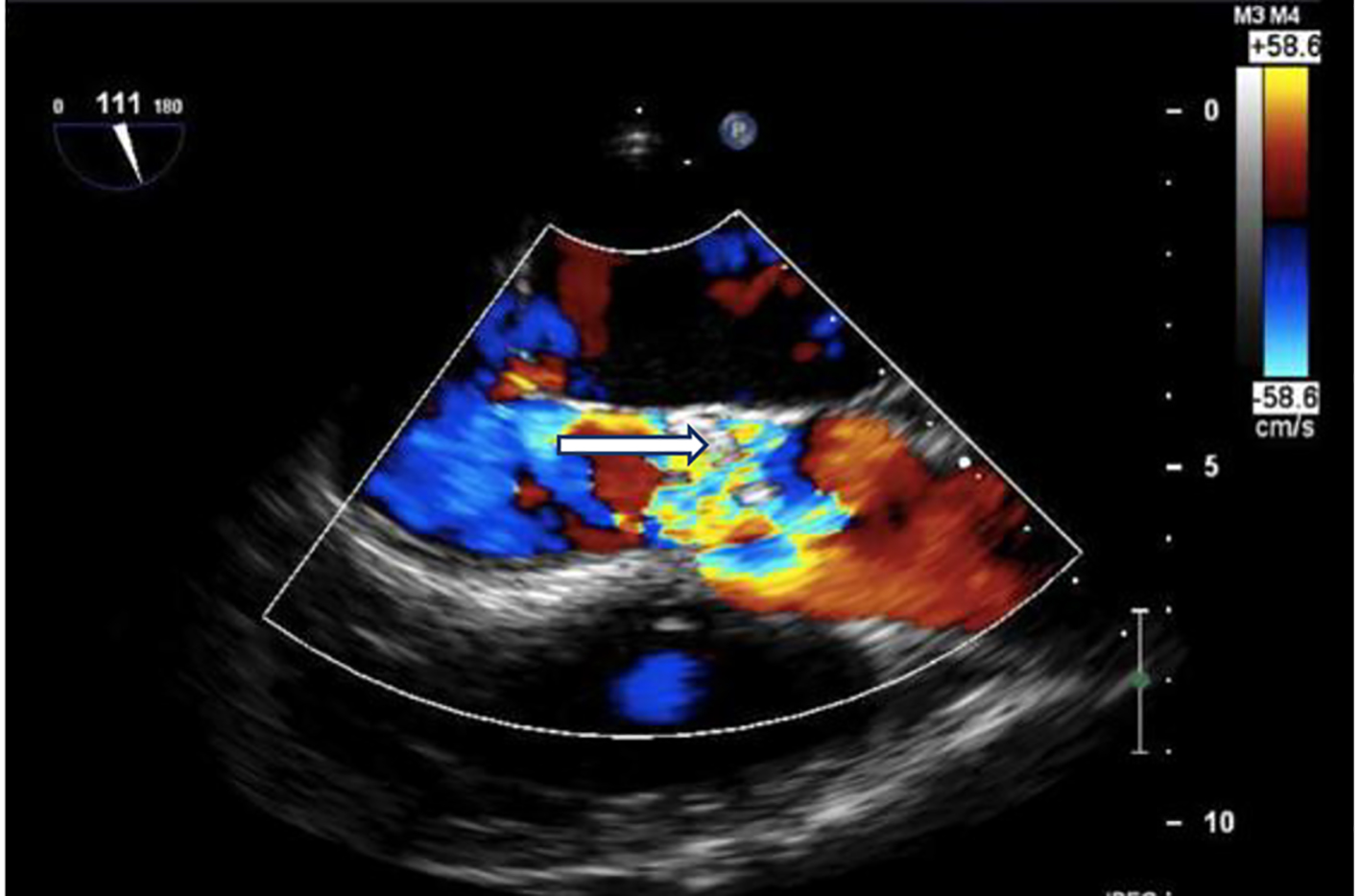 Click for large image | Figure 4. Echo: severe aortic valve regurgitation on colored Doppler flow (white arrow). |
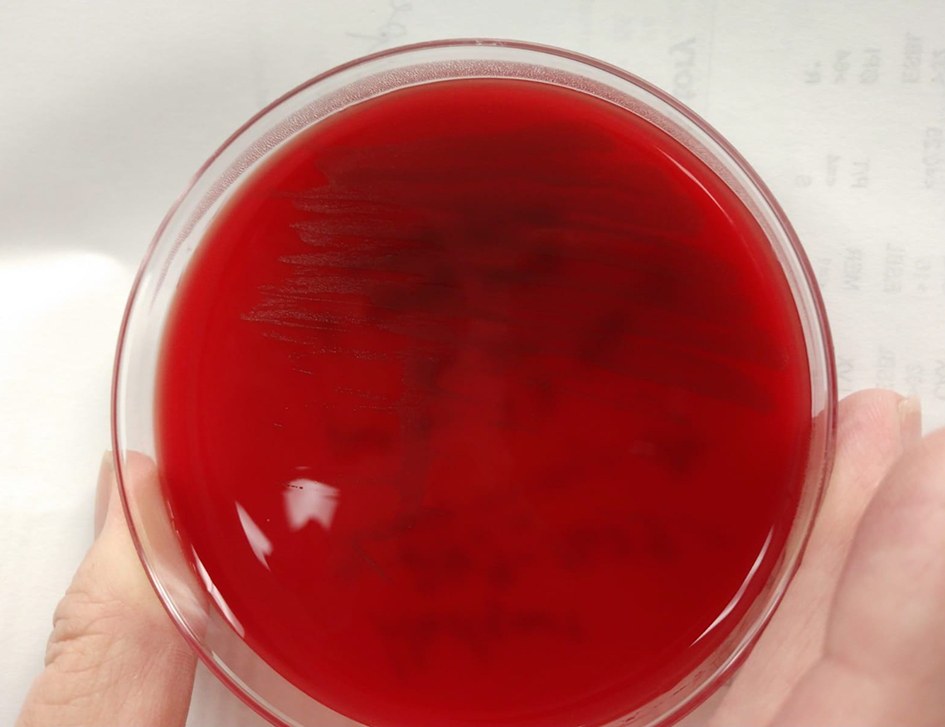 Click for large image | Figure 5. Isolate growing on blood agar, from our microbiology lab: University of South Alabama Health University Hospital. |
Unfortunately, the patient had an eventful hospital course. He developed pulmonary edema and required transfer to the intensive care unit for mechanical ventilatory support. Surgical AVR was performed (on hospital day 10) with a porcine bioprosthetic valve (Fig. 6). Anticoagulation with warfarin was started with a goal international normalized ratio (INR) of 2 - 3. Antibiotics were switched to vancomycin and nafcillin with the latter being eventually discontinued as the patient showed improvement. He received a total of 6 weeks of antibiotics therapy with vancomycin.
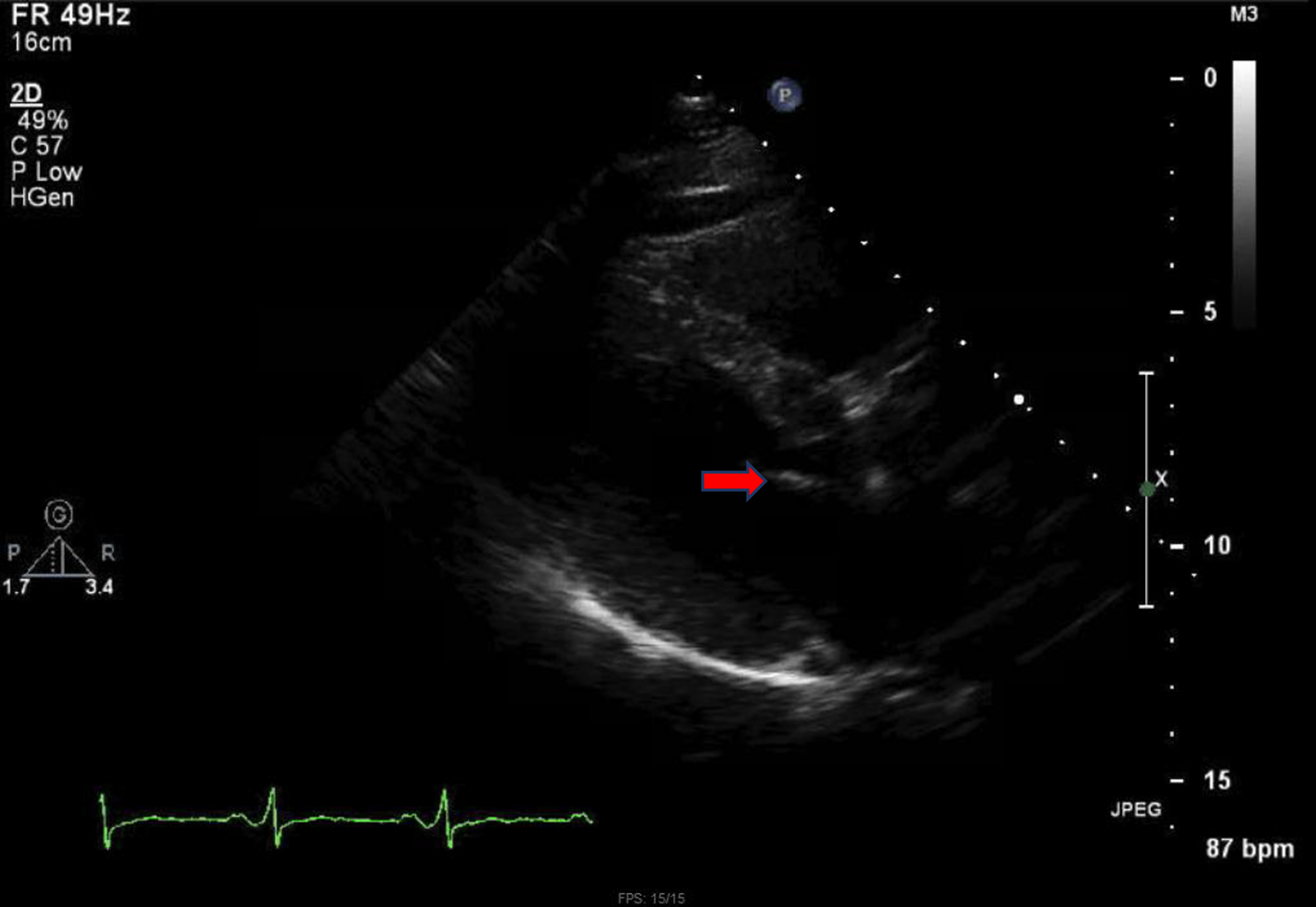 Click for large image | Figure 6. Normal structure and function of bioprosthetic valve (red arrow). |
| Discussion | ▴Top |
The incidence of UTI with AU is rare and the progression to an associated blood stream infection (BSI) is even less likely, which are estimated approximately 54 per 1,000,000/year for UTI and 3 per 1,000,000/year for BSI [5]. Less than 50 cases of AUE have been reported in literature so far [6]. AU accounts for approximately 0.2-0.8% of all UTIs [7, 8]. The most identified risk factors are underlying urologic conditions and old age [5, 9]. The urologic abnormalities include benign prostatic hyperplasia (BPH), urinary tract catheters, phimosis, urethral meatus stenosis, recurrent UTIs and genitourinary malignancy, with benign prostatic hyperplasia (BPH) being the most commonly associated abnormality given its prevalence [2, 7, 10, 11]. Kotkar et al reported a case that had phimosis as well as urethral meatus stenosis [11]. Our patient also had phimosis on presentation as well. Comorbid conditions such as diabetes mellitus, ischemic heart disease and tumors have also been described as risk factors [5, 12]. The average age of 29 patients in a literature review done in 2017 was 66.7 years [13], while it was 72 years for a review of 43 cases in 2018 [6]. There are very few cases of younger patients. Dysango et al reported two cases of patients aged 24 and 51 years [14].
Organism identification is challenging as it can sometimes be misidentified as other cocci on cultures. Once a culture results in a gram-positive, catalase-negative coccus that has alpha-hemolysis in blood agar, it is often terminated with the assumption that the causative agent is an alpha-hemolytic streptococcus [3]. Although aerococci appear as pairs, tetrads or in clusters like staphylococci, they do not produce catalase [8, 15]. They resemble streptococci in that they are alpha-hemolytic on blood agar plates, displaying small semi-transparent colonies (Fig. 5). AU grows under aerobic and anaerobic conditions [15]. It also forms biofilms by activating platelets on infected structures, which contributes to its pathogenesis and results in suboptimal host defense [16]. More rapid and accurate identification is possible with matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) [8]. Sequencing of the gene encoding 16SrRNA continues to remain the gold standard for identification of aerococci and separating them from other genera and each other [8, 17, 18]. However, this is impractical for routine clinical purposes as it is expensive, time-consuming and not available in all facilities. Biochemical characteristics are given in Table 1 [8, 16, 17].
 Click to view | Table 1. Biochemical Features of Aerococcus Species |
The presenting symptoms of AUE are similar to endocarditis with other pathogens and include fever, shortness of breath (potentially due to pulmonary edema from valvular dysfunction), malaise and in severe cases septic shock [19, 20]. Fever was present in all 16 patients in the study conducted by the Swedish Registry of Infective Endocarditis (SRIE) group. Fourteen patients in that registry had definite IE and two had possible IE, based on modified Duke criteria [18]. Our patient presented with altered mental status and imaging suggested cardioembolic stroke. Possible endocarditis was entertained in our patient based on the modified Duke criteria.
Complications reported in AUE are highly varied, mostly related to embolization. The first literature review done by Ebnother et al in 2002 reported the rate of embolization with AUE is higher as compared to other pathogens (55% or 6/11 patients) [21]. This phenomenon was reported to be 40% (12/29 patients) in a literature review in 2017 [13]. This might suggest an overestimation due to limited number of cases reported so far and the number will decline when more cases are reported. Embolization most commonly occurs to the central nervous system, resulting in neurologic deficits including hemiplegia [10, 22-24] or transient ischemic attack [25]. Embolization to the right coronary artery leading to an acute myocardial infarction has also been reported [11]. AUE leading to sepsis with multiorgan failure [18, 20, 21, 26, 27] and heart failure from mitral or aortic valve endocarditis causing significant regurgitation or stenosis have also been reported [7, 16, 28].
The treatment of aerococcal UTI and AUE is empiric and based on the cases reported so far as no formal guidelines exist due to the rarity of these cases and lack of clinical evidence. Very low minimum inhibitory concentrations (MICs) for penicillin and relatively low MICs for cephalosporins and carbapenems have been found for both A. urinae and A. Sanguinicola [6, 29]. The mainstay of treatment has been beta-lactams for AUE [30]. The most recent literature review in 2019 examined 45 patients and all patients received beta-lactams except for one case where treatment was not reported. It was mostly used in combination with other antibiotics, most common being an aminoglycoside (38/45 patients) due to its synergistic effect. The mean duration of treatment was 2 - 12 weeks [30]. Interestingly, the resistance of aerococcus species to aminoglycosides is either low or high, and AU shows the highest MICs, but a synergistic killing effect on two AUE isolates in vitro was found when penicillin was combined with netilmicin or gentamicin [6, 23, 29]. AU is intrinsically resistant to sulphonamides (like enterococci) and fluoroquinolones. Resistance to trimethoprim is also reported but that seems to be dependent on the medium used [3, 31-34]. Our patient had a very recent history of UTI treated empirically with trimethoprim/sulfamethoxazole as an outpatient, but no cultures were collected, and resistance likely explains failure of treatment. In vitro studies on AU demonstrate susceptibility to amoxicillin, cefotaxime, ceftriaxone, meropenem, linezolid, rifampin and vancomycin. There is variable resistance to clindamycin, erythromycin and levofloxacin [32, 33, 35]. The duration of treatment for UTI and phlegmon is 10 days, ranging from 14 to 28 days for BSI and 42 days for endocarditis [30]. Information on surgical or non-surgical management is provided in 26 cases only while 20 cases do not mention any information; 46.2% (12/26) had surgery while 53.8% (14/26) did not have surgical intervention [30]. A mortality of 50% (14/29) was reported in earlier literature review [13] but reported to be 28.2% (13/45) in the most recent review, which is still 27% higher than other causes of endocarditis [30] and assumed to be due to association with increased age and multiple comorbidities. In contrast, the prognosis was favorable in a study conducted by SRIE where all 16 patients survived the hospital stay. It is the largest study done so far, where 14 patients with A. urinae and two patients with A. sanguinicola endocarditis were studied [18]. The prognosis is favorable in patients who underwent surgery compared to those who did not (80% survived). Interestingly, all the patients who received beta-lactam as monotherapy in combination with surgery survived [30].
Conclusion
AU is a rare etiology of IE with limited number of cases reported in literature to guide its diagnosis and management. The rate of complications, such as embolization, and the associated mortality is reported higher for AUE. We believe this is due to difficulties in correct diagnosis, proper pathogen identification, patients being older and having multiple comorbidities. There might be an overestimation of these outcomes due to only complicated and challenging cases being reported. The actual incidence might be underestimated. When promptly and properly identified and appropriate treatment initiated, the prognosis of AUE can be favorable with medical therapy alone or combined with surgery. The goal of this case report is to add important data to the literature regarding AUE, with specific aim towards highlighting its low incidence, risk factors, variability of presentation, challenges in diagnosis and treatment options to consider as there are no clinical data available to guide management.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
An informed consent has been obtained by the author from the patient prior to submission of this work.
Author Contributions
Yasir Ahmed drafted abstract, case presentation and discussion, and tables and pictures and references, and also corresponded with the supervisor and finalized the report for submission. Nikky Bardia contributed to drafting, introduction and discussion and its critical analysis, and final proofreading and approval. Caleb Judge contributed to discussion and its critical analysis and confirmed lab values. Supervisor: Dr. Eduardo Calderon (Associate Professor Internal Medicine and Infectious Diseases). Sajjad Ahmad contributed to discussion, analysis, reading images and reporting and input from cardiology perspective. Supervisor: Dr. Christopher Malozzi (Assistant Professor Department of Cardiology).
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Zhang Q, Kwoh C, Attorri S, Clarridge JE, 3rd. Aerococcus urinae in urinary tract infections. J Clin Microbiol. 2000;38(4):1703-1705.
doi pubmed - Buchbinder L, Solowey M, Solotorovsky M. Alpha hemolytic streptococci of air: their variant forms, origin and numbers per cubic foot of air in several types of locations. Am J Public Health Nations Health. 1938;28(1):61-71.
doi pubmed - Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480.
doi pubmed - Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405.
doi pubmed - de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447.
doi pubmed - Yabes JM, Perdikis S, Graham DB, Markelz A. A rare case of Aerococcus urinae infective endocarditis in an atypically young male: case report and review of the literature. BMC Infect Dis. 2018;18(1):522.
doi pubmed - Rasmussen M. Aerococcus: an increasingly acknowledged human pathogen. Clin Microbiol Infect. 2016;22(1):22-27.
doi pubmed - Rasmussen M. Aerococci and aerococcal infections. J Infect. 2013;66(6):467-474.
doi pubmed - Serefhanoglu K, Turan H, Dogan R, Gullu H, Arslan H. A case of Aerococcus urinae septicemia: an unusual presentation and severe disease course. Chin Med J (Engl). 2005;118(15):1318-1320.
- Kass M, Toye B, Veinot JP. Fatal infective endocarditis due to Aerococcus urinae—case report and review of literature. Cardiovasc Pathol. 2008;17(6):410-412.
doi pubmed - Kotkar KD, Said SM, Michelena H, Wanta B, Fritock MD, Baddour LM. Right Coronary Artery Septic Embolization Secondary to Aerococcus urinae Native Mitral Valve Endocarditis. Ann Thorac Surg. 2016;102(4):e295-297.
doi pubmed - Grude N, Jenkins A, Tveten Y, Kristiansen BE. Identification of Aerococcus urinae in urine samples. Clin Microbiol Infect. 2003;9(9):976-979.
doi pubmed - Adomavicius D, Bock M, Vahl CF, Siegel E. Aerococcus urinae Mitral Valve Endocarditis-Related Stroke: A Case Report and Literature Review. J Investig Med High Impact Case Rep. 2018;6:2324709618758351.
doi pubmed - Dysangco A, Coronel RF, Li-Yu J, Purino FM, Sunarso S. Aerococcus urinae endocarditis: a report of two cases and review of literature. Philipp J Intern Med. 2010;48:49-52.
- Shannon O, Morgelin M, Rasmussen M. Platelet activation and biofilm formation by Aerococcus urinae, an endocarditis-causing pathogen. Infect Immun. 2010;78(10):4268-4275.
doi pubmed - Lawson PA, Falsen E, Truberg-Jensen K, Collins MD. Aerococcus sanguicola sp. nov., isolated from a human clinical source. Int J Syst Evol Microbiol. 2001;51(Pt 2):475-479.
doi pubmed - Lawson PA, Falsen E, Ohlen M, Collins MD. Aerococcus urinaehominis sp. nov., isolated from human urine. Int J Syst Evol Microbiol. 2001;51(Pt 2):683-686.
doi pubmed - Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173.
doi pubmed - Senneby E, Goransson L, Weiber S, Rasmussen M. A population-based study of aerococcal bacteraemia in the MALDI-TOF MS-era. Eur J Clin Microbiol Infect Dis. 2016;35(5):755-762.
doi pubmed - Melnick S, Nazir S, Hingorani R, Wexler P. Aerococcus urinae, a rare cause of infective endocarditis. BMJ Case Rep. 2016;2016.
doi pubmed - Ebnother C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313.
doi pubmed - Christensen JJ, Gutschik E, Friis-Moller A, Korner B. Urosepticemia and fatal endocarditis caused by aerococcus-like organisms. Scand J Infect Dis. 1991;23(6):717-721.
doi pubmed - Creed JA, Patel D, Goldstein LB. Aerococcus Urinae infective endocarditis-related stroke: a case report. J Cardiovasc Dis Diagn. 2016;4:232.
- Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124.
doi pubmed - Gritsch W, Nagl M, Hausdorfer J, Gschwendtner A, Pechlaner C, Wiedermann CJ. Septicaemia and endomyocarditis caused by Aerococcus urinae. Wien Klin Wochenschr. 1999;111(11):446-447.
- Georgescu CL, Zbinden R, Herren T. Schwere Aerococcus-urinae-endokarditis. Schweiz Med Forum. 2004;4:1073-1074.
doi - Westmoreland K, Halstead DC, DuBose PV. Infectious endocarditis in 49-year-old man and discussion of phenotypic characteristics of Aerococcus Urinae and Viridans Streptococci. Lab Med. 2014;45(3):e101-103.
doi pubmed - Skov R, Christensen JJ, Korner B, Frimodt-Moller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658.
doi pubmed - Facklam R, Lovgren M, Shewmaker PL, Tyrrell G. Phenotypic description and antimicrobial susceptibilities of Aerococcus sanguinicola isolates from human clinical samples. J Clin Microbiol. 2003;41(6):2587-2592.
doi pubmed - Figueroa Rodriguez F, Faieta Lasarcina A, Davila Grijalva F. Mitral valve endocarditis with perforation from a urinary source: an unusual case and literature review. Case Rep Cardiol. 2019;2019:5496851.
doi pubmed - Humphries RM, Lee C, Hindler JA. Aerococcus urinae and trimethoprim-sulfamethoxazole. J Clin Microbiol. 2011;49(11):3934-3935.
doi pubmed - Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875.
doi pubmed - Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100S, 26th edition, 2016.
- Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276.
doi pubmed - Haggstrom MJ. Microscopy of Aerococcus urinae with gram stain, showing gram positive cocci. Images of Aerococcus urinae. WikiJournal of Medicine. 2015;2(1).
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


