| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 12, Number 11, November 2021, pages 446-450
Obliterative Portal Venopathy Caused by Oral Contraceptive Pills: A Case Report
Muhammad Farhan Ashrafa, d, Radiana Trifonovab, Asra Batoolc
aDepartment of Internal Medicine, Albany Medical Center, NY, USA
bDepartment of Pathology, Albany Medical Center, NY, USA
cDivision of Gastroenterology, Albany Medical Center, NY, USA
dCorresponding Author: Muhammad Farhan Ashraf, Department of Internal Medicine, Albany Medical Center Hospital, Albany, NY 12208, USA
Manuscript submitted August 22, 2021, accepted September 22, 2021, published online November 5, 2021
Short title: Obliterative Portal Venopathy Caused by OCPs
doi: https://doi.org/10.14740/jmc3779
| Abstract | ▴Top |
Oral contraceptive pills (OCPs) have a known prothrombotic effect. Obliterative portal venopathy (OPV) can be seen in patients with underlying hypercoagulability. We present a case of a 19-year-old female patient taking OCPs who presented with obstructive jaundice. Her main concern was pruritis. An extensive workup was done to reach a diagnosis but it came back negative. A liver biopsy showed OPV. This was thought secondary to her OCP use. Her OCPs were discontinued which resulted in a complete resolution of her symptoms and laboratory abnormalities. Cases with a direct relationship between OPV and OCP use are extremely rare. More studies are required to establish a correlation between OPV and OCPs. OPV should be considered in the differential diagnosis among patients with obstructive jaundice without an obvious cause, especially in patients taking OCPs. Treatment is stopping the OCPs with close follow-up to confirm disease resolution.
Keywords: Obliterative portal venopathy; Idiopathic noncirrhotic portal hypertension; Hepatoportal sclerosis; Oral contraceptive pills; Gastroenterology; Obstructive jaundice
| Introduction | ▴Top |
Combined oral contraceptive pills (OCPs) are generally safe but can cause side effects like the development of prothrombotic conditions. This is because estrogen increases the level of fibrinogen and coagulation factors like factors II, VII, VIII, and X [1]. Estrogen is also linked with a decrease in levels of antithrombin, protein S, and acquired resistance to protein C. Historically, estrogen was thought to be the active component in combined OCPs responsible for their prothrombotic effects; but now it is clear that progestins also play an important role. Levonorgestrel causes more activated protein C resistance than desogestrel. Also, desogestrel increases procoagulant factors VII, VIII, and X while decreasing levels of protein S and antithrombin [2]. Hepatic synthesis of fibrinogen, factor VII and X are increased due to first-pass hepatic metabolism of both estrogen and progestins. Obliterative portal venopathy (OPV) is the term used for primary occlusion of intrahepatic portal veins in noncirrhotic patients in the absence of intrahepatic inflammation or neoplasia. The prevalence of OPV is unknown in the general population. A study in 2016 including 482 patients with noncirrhotic cryptogenic chronic liver disease who underwent liver biopsy showed OPV in 19.5% of these patients [3]. OPV is poorly understood since it lacks pathognomonic clinical features. It is a subtype of noncirrhotic portal hypertension (NCPH) which itself is rare in the USA but is relatively common in India [4]. The etiopathogenesis of OPV is unknown and several hypotheses are proposed including antiretroviral therapy, hypercoagulability, genetic predisposition, recurrent bacterial infections, and chemical/toxin exposure [5]. Definitive diagnosis is based on biopsy results. Histopathology usually shows several portal vein abnormalities including vein herniation outside of the portal tract border into the hepatic parenchyma and its replacement by small vein radicles. Intimal fibrous thickening and collagen deposition into the space of Disse is also seen [6].
It has been hypothesized that OCPs can also cause OPV with underlying hypercoagulability is one of its proposed etiologies [7, 8]. No dedicated case reports or studies are found in the literature indicating a link between OCPs and OPV. We report a case of biopsy-proven OPV causing large bile duct obstruction picture from combined OCP use where slow resolution occurred after discontinuation of the medication.
| Case Report | ▴Top |
Investigations
A 19-year-old Caucasian woman with a past medical history of Down’s syndrome and hypothyroidism presented with a 4-day history of progressively worsening jaundice, pruritus and right upper quadrant abdominal pain. The patient also complained of poor appetite, dark urine and pale stools. She also complained of fatigue for the past month. She was particularly concerned about pruritus which was not relieved by over-the-counter medications. Her abdominal pain had no relationship with her food intake. She denied any fevers, alcohol use, recent travel, sick contacts, or use of herbal supplements. Her medications included levothyroxine, fexofenadine and levonorgestrel-ethinyl estradiol combined OCPs. She was sexually active and had started taking the OCPs 6 months before the presentation. She had no history of any pregnancies. The patient was a non-smoker and did not consume any alcohol. She had no personal or family history of liver and biliary disorders. Family history was also negative for any genetic disorders or gastrointestinal malignancies. The patient reported difficulty with losing weight, her body mass index (BMI) was 40.8 kg/m2.
Physical exam was remarkable for scleral icterus and right upper quadrant tenderness with positive Murphy’s sign. There was no rebound tenderness or peritoneal signs.
Diagnosis
Labs showed total bilirubin of 8.0 mg/dL with a direct bilirubin of 5.1 mg/dL, alkaline phosphatase of 245 IU/L, aspartate transaminase (AST) of 50 IU/L, alanine aminotransferase (ALT) of 100 IU/L, erythrocyte sedimentation rate (ESR) of 10 mm/h, international normalized ratio (INR) of 1.1 and lipase of 23 IU/L. Differential diagnoses included cholangitis, choledocholithiasis, acute hepatitis secondary to various etiologies and drug toxicity. Acute and chronic hepatitis workup was negative for acetaminophen toxicity, viral hepatitis, Epstein-Barr virus (EBV), cytomegalovirus (CMV) and herpes simplex virus (HSV) serologies, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, Wilson’s disease, hemochromatosis, α1 antitrypsin deficiency and human immunodeficiency virus (HIV) infection. The urine toxicology screen was negative as well. Ultrasound showed no evidence of cholecystitis, cholelithiasis or choledocholithiasis. There was no intra or extrahepatic biliary obstruction. Computed tomography (CT) scan and magnetic resonance cholangiopancreatography (MRCP) were unremarkable. Alkaline phosphatase progressively increased to 500 IU/L, AST was increased to 78 IU/L and ALT was elevated to 195 IU/L. The major challenge faced at this point was that extensive workup as mentioned above was unable to reach a final diagnosis while the patient’s condition and labs were deteriorating. It was decided at this point to perform a liver biopsy.
The liver biopsy showed findings of large bile duct obstruction and obstructive portal venopathy (OPV, Fig. 1). Endoscopic ultrasound (EUS) was performed but was unremarkable for any biliary obstruction. A diagnosis of OPV secondary to OCP use in absence of other etiologies was made.
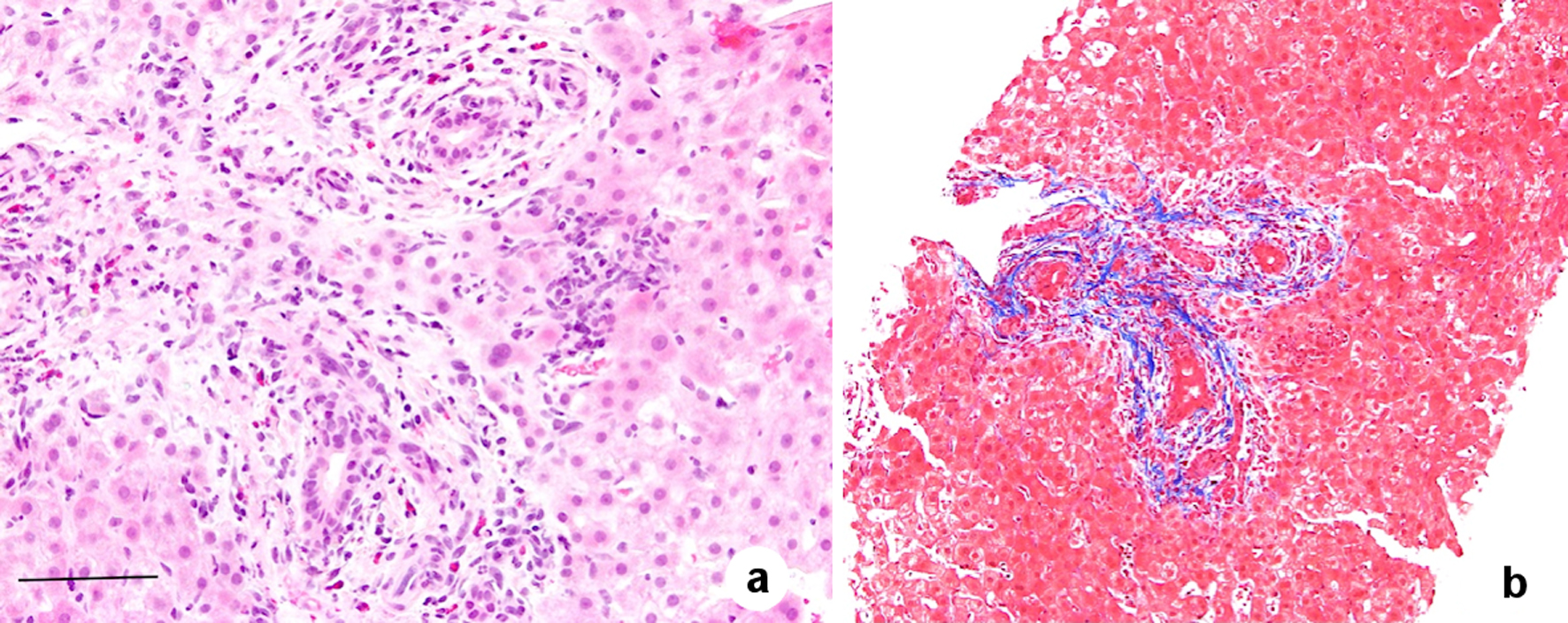 Click for large image | Figure 1. Histological features of obliterative portal venopathy including fibrotic portal tracts with the absent normal portal vein. There is no bridging fibrosis and no evidence of cirrhosis (trichrome stain). In addition, biliary duct injury with eosinophil reaction in the portal area is present. Hematoxylin and eosin (H&E) staining, magnification × 200, scale bar = 20 µm (a); and trichrome stain, magnification × 100 (b). |
Treatment
The patient was advised to stop taking her OCPs and consider an alternative contraceptive method. A follow-up appointment with the gastroenterology clinic was made within a month after discharge.
Follow-up and outcomes
On 3-week follow-up after discharge from the hospital, the patient’s labs and symptoms significantly improved. The physical exam showed no icterus and the abdominal exam was completely benign. Liver enzymes and bilirubin improved with normalization in the next 5 months. Repeat bilirubin was 0.7 mg/dL, AST was 32 IU/L and ALT was 44 IU/L. There were no unanticipated or adverse events. The case timeline is described in Figure 2.
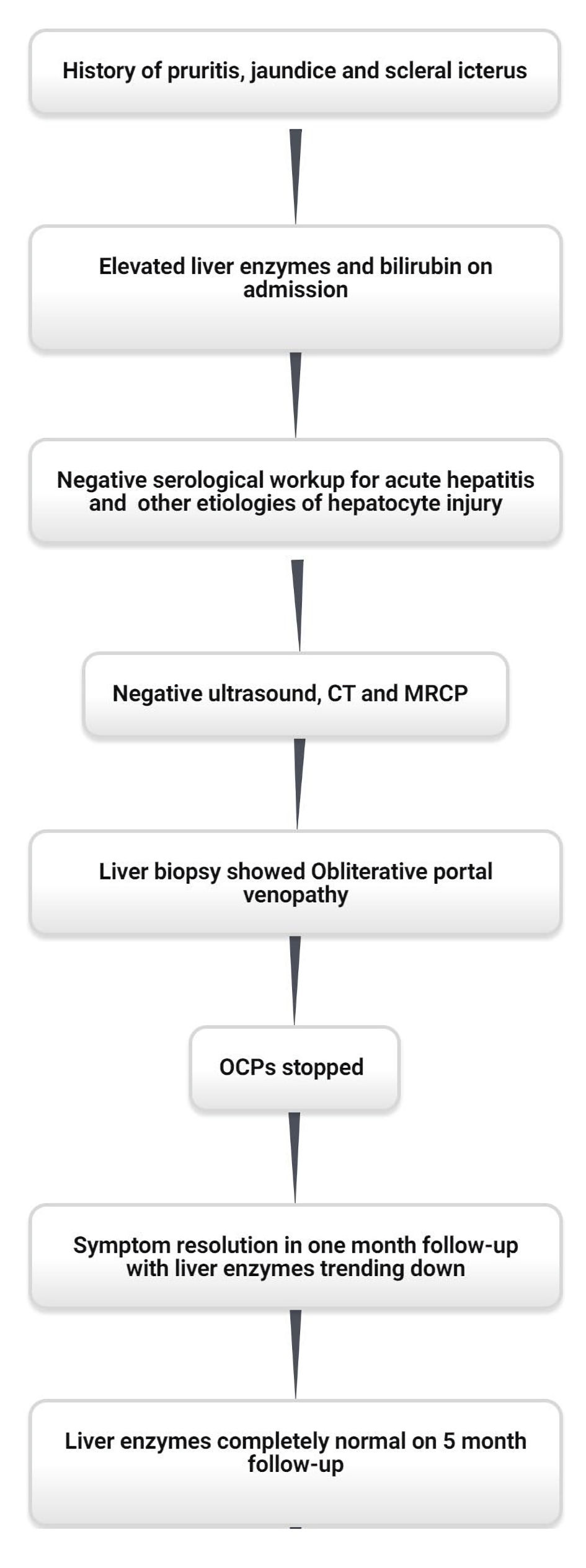 Click for large image | Figure 2. Case timeline. CT: computed tomography; MRCP: magnetic resonance cholangiopancreatography; OCPs: oral contraceptive pills. |
| Discussion | ▴Top |
Nearly all women use contraception at some point in their lifetime. According to the National Health Statistics Report (NHSR) published by the Center for Disease Control (CDC) in 2017 - 2019, 65.3% of women aged 15 - 49 were using contraception. Of these women, 14% women were using OCPs [9]. OCPs are notoriously known to increase a patient’s tendency to develop worrisome coagulopathies including venous thromboembolism, myocardial infarction (MI), and thrombotic stroke [10-12]. Although this phenomenon is much more common in women who are older than 35 years and smoke, this can occur in any patient taking OCPs. Estrogen-containing OCPs increase coagulation by increasing the level of plasma fibrinogen and action of individual coagulation factors including factors VII and X, while also dampening the anticoagulant protective mechanisms by decreasing antithrombin III. Research shows that the level of coagulation correlates with the estrogen dose in the oral contraceptive itself. While deemed mostly safe, they can cause hepatic and biliary dysfunction and rarely women can develop intrahepatic cholestasis. Liver biopsy commonly shows dilatation of bile canaliculi, bile stasis, shortening and blunting of microvilli, but OPV is not common.
Also called hepatoportal sclerosis, OPV is one of the subtypes of NCPH [13]. According to one study, 19.5% of patients with noncirrhotic cryptogenic liver disease had OPV on biopsy [3]. Patients with NCPH often have various presentations which can include ascites, encephalopathy, and hepatosplenomegaly. However, some patients may develop more severe symptoms with advanced disease, which can manifest with acute variceal bleeding or hepatopulmonary syndrome [14]. There are several possible etiologies of OPV, but it is most commonly seen in patients with collagen vascular disease, hematologic pathologies such as primary thrombocytopenia, polycythemia vera, multiple myeloma and chronic use of various medications including azathioprine and didanosine [13]. Other less common causes of OPV include genetic susceptibility, exposure to toxic substances, repeated bacterial infections, and treatment of HIV (didanosine). Finally, OPV can rarely arise in patients in a hypercoagulable state such as our patient who used OCP. However, most cases of OPV do not have a clear etiology and are often labeled as idiopathic [14].
The presentation of OPV is non-specific and may present with either a large bile duct obstruction as in our patient or portal hypertension. In some cases of OPV, patients also have portal venous thrombosis which can be seen on imaging. In advanced OPV, the liver is indistinguishable from cirrhosis while in the early stages, parenchyma can be normal. On imaging, a wide range of intrahepatic and/or extrahepatic portal venous abnormalities can be seen with changes in liver and spleen stiffness and volume [15]. In our case imaging was completely unremarkable which is interesting. The liver was normal in size and contour and the portal vein was patent. OPV is definitively diagnosed by biopsy, which classically demonstrates thrombosis and sclerosis of the portal venous system, generation of minor vascular passages inside and circumferential to the portal tracts, and fibrosis. It is believed that this pattern of vascular disease is from diffuse thrombotic lesions throughout the tract, and the nodular regenerative hyperplasia is an adverse consequence of this process [16]. OPV and NCPH generally have a benign disease course but sometimes may cause hepatic failure, encephalopathy and portal vein thrombosis. Anticoagulation should be considered in patients who develop portal vein thrombosis as a result. Since OCPs use increases the risk for thrombosis as mentioned in the mechanisms described above, they may lead to the pathogenesis of OPV. The case presented above is an extremely rare occurrence and more studies are needed to establish a correlation between OCP use and the development of OPV. Although portal venous thrombosis has been reported with OCP use in a single case report, no cases of intrahepatic OPV related to OCP use have been reported so far [17].
Learning points
OPV is a rare and under-reported entity. Through this case, we want to highlight that OCPs can cause liver injury which can present as OPV. This is due to the prothrombotic effects of OCPs and should be considered as a differential diagnosis if the labs and biopsy show findings suggestive of large bile duct obstruction. A liver biopsy may be required in these cases to establish a definitive diagnosis. OCPs should be stopped if there is suspicion of OPV in patients taking OCPs. In our case, stopping the medication led to the complete resolution of symptoms.
Acknowledgments
None to declare.
Financial Disclosure
There was no financial support provided by any institution or industry.
Conflict of Interest
There is no conflict of interest to be reported in this case report by any of the authors.
Informed Consent
Informed consent from the patient was obtained for publication of the case.
Author Contributions
MFA served as the primary author of this case report and worked on all the elements right from the introduction to the discussions. RT contributed to the pathological aspect of the case. AB provided oversight in the case and contributed to the discussion. All authors of this case report had important contributions that led to its final form.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Conard J. Biological coagulation findings in third-generation oral contraceptives. Hum Reprod Update. 1999;5(6):672-680.
doi pubmed - Oger E, Alhenc-Gelas M, Lacut K, Blouch MT, Roudaut N, Kerlan V, Collet M, et al. Differential effects of oral and transdermal estrogen/progesterone regimens on sensitivity to activated protein C among postmenopausal women: a randomized trial. Arterioscler Thromb Vasc Biol. 2003;23(9):1671-1676.
doi pubmed - Guido M, Sarcognato S, Sonzogni A, Luca MG, Senzolo M, Fagiuoli S, Ferrarese A, et al. Obliterative portal venopathy without portal hypertension: an underestimated condition. Liver Int. 2016;36(3):454-460.
doi pubmed - Goel A, Ramakrishna B, Zachariah U, Sajith KG, Burad DK, Kodiatte TA, Keshava SN, et al. What makes non-cirrhotic portal hypertension a common disease in India? Analysis for environmental factors. Indian J Med Res. 2019;149(4):468-478.
doi pubmed - Schouten JN, Garcia-Pagan JC, Valla DC, Janssen HL. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011;54(3):1071-1081.
doi pubmed - Gonzalez RS. Hepatoportal sclerosis. 2021. [cited August 19th, 2021]. Available from: https://www.pathologyoutlines.com/topic/liverhepatoportalsclerosis.html.
- Sarin SK. Non-cirrhotic portal fibrosis. J Gastroenterol Hepatol. 2002;17(Suppl 3):S214-223.
doi pubmed - Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D, Valla D, et al. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51(2):275-280.
doi pubmed - Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;388:1-8.
- Amoozegar F, Ronksley PE, Sauve R, Menon BK. Hormonal contraceptives and cerebral venous thrombosis risk: a systematic review and meta-analysis. Front Neurol. 2015;6:7.
doi pubmed - Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120(7):801-810.
doi pubmed - Peragallo Urrutia R, Coeytaux RR, McBroom AJ, Gierisch JM, Havrilesky LJ, Moorman PG, Lowery WJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122(2 Pt 1):380-389.
doi pubmed - Agrawal M, Rahmani R, Nakkala K, Fiel MI, Schiano T. Hepatoportal sclerosis (obliterative portal venopathy) and nodular regenerative hyperplasia in a patient with myasthenia gravis: A case report and review of the published work. Hepatol Res. 2013;43(9):999-1003.
doi pubmed - Aggarwal S, Fiel MI, Schiano TD. Obliterative portal venopathy: a clinical and histopathological review. Dig Dis Sci. 2013;58(10):2767-2776.
doi pubmed - Arora A, Sarin SK. Multimodality imaging of obliterative portal venopathy: what every radiologist should know. Br J Radiol. 2015;88(1046):20140653.
doi pubmed - Wanless IR, Godwin TA, Allen F, Feder A. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore). 1980;59(5):367-379.
doi - Chu G, Farrell GC. Portal vein thrombosis associated with prolonged ingestion of oral contraceptive steroids. J Gastroenterol Hepatol. 1993;8(4):390-393.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


