| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 12, Number 12, December 2021, pages 474-480
Successful Treatment of Thrombocytopenia, Anasarca, Fever, Reticulin Myelofibrosis/Renal Insufficiency, and Organomegaly Syndrome Using Plasma Exchange Followed by Rituximab in the Intensive Care Unit
Yusuke Otsukaa, Akihiro Shirakabea, c, Toshio Asayamab, Hirotake Okazakia, Yusaku Shibataa, Shota Shigiharaa, Tomofumi Sawatania, Norio Yokoseb, Kuniya Asaia
aDivision of Intensive Care Unit, Nippon Medical School Chiba Hokusoh Hospital, Chiba, Japan
bDepartment of Hepatology, Nippon Medical School Chiba Hokusoh Hospital, Chiba, Japan
cCorresponding Author: Akihiro Shirakabe, ICU, Nippon Medical School Chiba Hokusoh Hospital, 1715 Kamagari, Inzai, Chiba 270-1694, Japan
Manuscript submitted September 6, 2021, accepted October 9, 2021, published online December 2, 2021
Short title: Treatment of TAFRO by PE and Rituximab
doi: https://doi.org/10.14740/jmc3784
| Abstract | ▴Top |
Thrombocytopenia, anasarca, fever, reticulin myelofibrosis/renal insufficiency, and organomegaly (TAFRO) syndrome is treated using corticosteroids and/or immunosuppressive agents as first-line therapy. We report the case of a 69-year-old female with TAFRO syndrome in which the patient presented multiple organ failure and steroid resistance, which was successfully treated using plasma exchange (PE) followed by rituximab. Decisions regarding the next treatment, including PE, are urgent for patients with steroid-resistant TAFRO syndrome. Since it is considered that immunosuppressive agents may be removed by PE, the performance of PE before treatment with immunosuppressive agents might be an option for steroid-resistant TAFRO syndrome.
Keywords: Castleman’s disease; Mechanical support; Intensive care
| Introduction | ▴Top |
TAFRO syndrome was first reported in 2010. It is a systemic inflammatory disorder named based on the following conditions: thrombocytopenia (T), anasarca (A), fever (F), reticulin fibrosis, renal failure (R) and organomegaly (O) [1]. Since the lymph node pathology and clinical course are similar to idiopathic multicentric Castleman’s disease (iMCD), it was initially recognized as a variant of MCD [2]. However, Fujimoto et al demonstrated in recent study that it had similar clinical course (long-term prognostic impact) between the TAFRO syndrome without lymph node pathology and the TAFRO syndrome with iMCD histopathology [2]. Diagnostic criteria was finally updated in 2021 [3], and concepts of TAFRO syndrome were suggested as following: 1) TAFRO syndrome with lymph node histopathology consistent with iMCD (iMCD-TAFRO); 2) TAFRO syndrome with no lymph node biopsy performed and no other comorbidities (possible iMCD-TAFRO); 3) TAFRO syndrome with lymph node histopathology not consistent with iMCD or other comorbidities (TAFRO without iMCD or other comorbidities). TAFRO syndrome is now diagnosed regardless of whether iMCD or not. TAFRO syndrome is often difficult to diagnose rapidly and there is currently no definitive treatment strategy; thus, in some cases, TAFRO syndrome follows a fatal course. The treatment protocol is decided on a case-by-case basis. According to case reports and experience, corticosteroids are recommended as first-line therapy, while immunosuppressive agents (e.g., cyclosporin A, tocilizumab, and rituximab) are usually recommended as second-line treatments. The effects of these treatments were evaluated using the severity classification, which was scored according to the scores for anasarca, thrombocytopenia, fever and renal failure. Each index is scored from grade 0 to 3, with the grade classification determined by the total score (grade 1, score 0 - 4; grade 2, score 5 - 6; grade 3, score 7 - 8; grade 4, score 9 - 10; and grade 5, score 11 - 12) [4]. Although most cases are treated by combined therapy with corticosteroids and immunosuppressive agents, other options are suggested for refractory cases. Plasma exchange (PE) has been reported to be effective in case reports for removing unknown pathogenic substances associated with this syndrome, including cytokines (e.g., interleukin-6 (IL-6), vascular endothelial growth factor (VEGF)), antigen-complexes and antibodies [5]. We herein report a case of TAFRO syndrome in which the patient presented multiple organ failure and steroid resistance, which was successfully treated using PE followed by rituximab.
| Case Report | ▴Top |
Investigations
A 69-year-old female visited the emergency room with fever that had persisted for 2 weeks. Computed tomography (CT) on the same day revealed multiple lymphadenopathy in the abdominal cavity. A blood examination revealed the exacerbation of renal and liver function marker levels within several days, and she was admitted to hospital. Time course of her laboratory data after admission were noted in Table 1. Although antibiotics (ceftriaxone to meropenem) were administered during the first week after admission, her inflammatory markers remained elevated and her fever persisted. Anasarca with fluid retention (i.e., pleural effusion and ascites) showed extreme progression after admission and a blood examination revealed thrombocytopenia.
 Click to view | Table 1. Time Course of Her Laboratory Data |
Diagnosis
As her condition progressed, she satisfied the three major diagnostic criteria (anasarca with fluid retention, thrombocytopenia, and fever with high inflammatory marker levels) and two minor diagnostic criteria (multiple lymphadenopathy and renal dysfunction) for TAFRO syndrome.
Treatment
Diuretics (intravenous furosemide and oral administration of tolvaptan) had no effect on her fluid retention. Steroid pulse therapy followed by steroid maintenance therapy was performed to treat TAFRO syndrome, and hemodialysis was performed in an attempt to improve her fluid retention (Fig. 1). However, these strategies were ineffective, and her anasarca with fluid retention worsened. Finally, she was admitted to the intensive care unit (ICU) on the 24th day after admission. Mechanical ventilation support was required after endotracheal intubation due to respiratory failure, and continuous renal replacement therapy (CRRT) was performed due to renal failure. Since her liver function had not recovered after these treatments, her inflammatory marker levels remained elevated and her fever persisted, we decided to perform PE (albumin replacement for 2 days and fibrinogen replacement for 4 days) as a treatment for TAFRO syndrome. The rapid initiation of PE was very effective. Her fever quickly declined, her inflammatory reaction decreased, and her anasarca with fluid retention showed a dramatic improvement. After the beneficial effects of PE, rituximab (every week) was initiated.
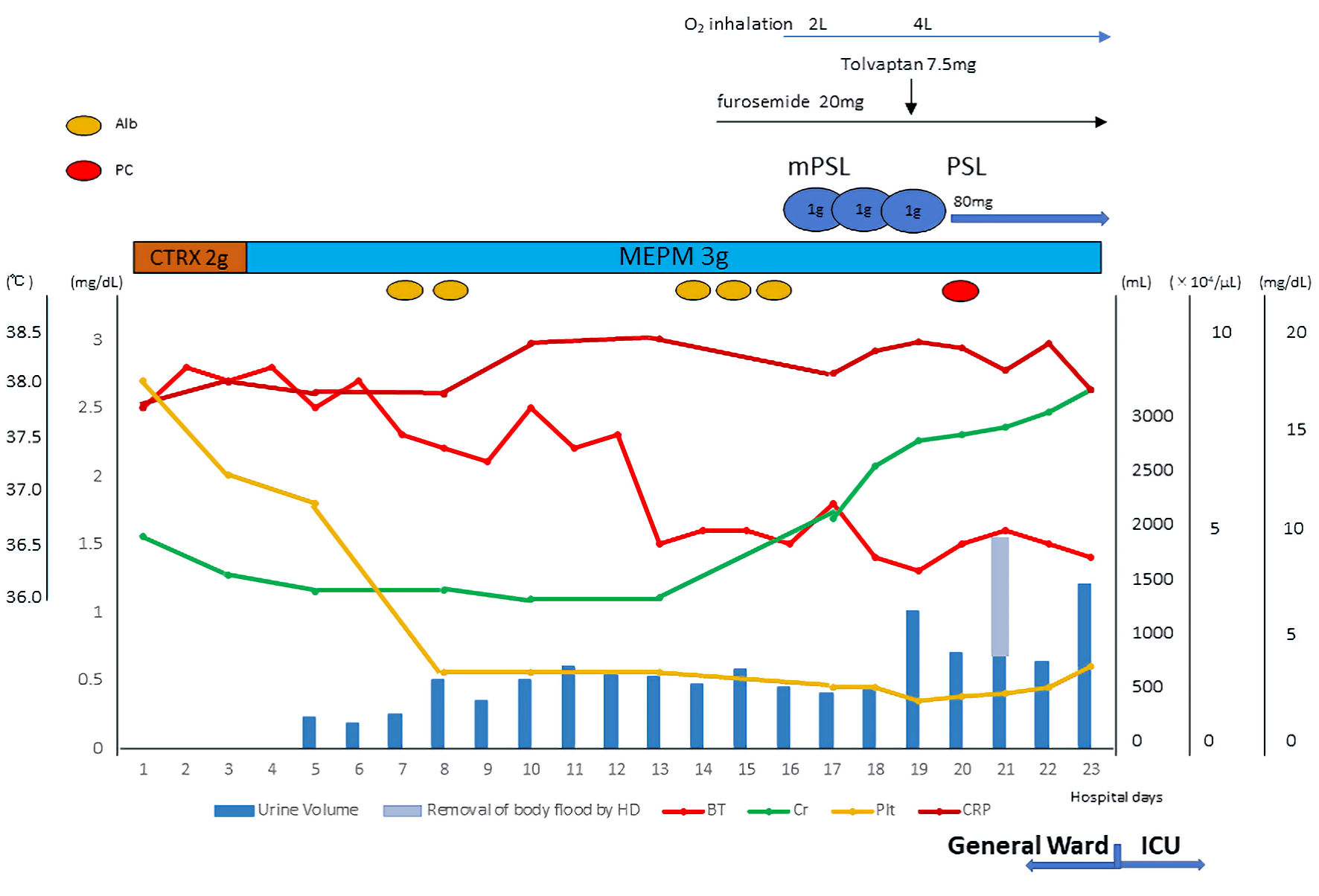 Click for large image | Figure 1. Time course of treatment in the general ward. Antibiotics were administered during the first week after admission; however her inflammatory markers remained elevated and her fever persisted. Anasarca with fluid retention showed extreme progression, and a blood examination revealed thrombocytopenia and developed renal dysfunction. She was diagnosed as TAFRO syndrome. Diuretics had no effect on her fluid retention, steroid pulse therapy followed by steroid maintenance therapy and hemodialysis was performed in an attempt to improve her fluid retention. However, no therapeutic effect was obtained. Finally, she was admitted to the ICU. TAFRO: thrombocytopenia, anasarca, fever, reticulin myelofibrosis/renal insufficiency, and organomegaly; HD: hemodialysis; PSL: prednisolone; CTRX: ceftriaxone; MEPM: meropenem; BT: body temperature; Cr: creatinine; Plt: platelet; ICU: intensive care unit. |
Follow-up and outcomes
Respiratory support was smoothly withdrawn and the patient was transferred to the general ward on the 48th day after admission (Figs. 2, 3). Rituximab treatment was performed for 1 month (four times every 1 week), and her good general condition was maintained. After 3 months of multidisciplinary treatment she made a complete recovery and was discharged to a rehabilitation hospital. We are planning to use the tocilizumab if she required the re-admission by the recurrence of TAFRO syndrome in future.
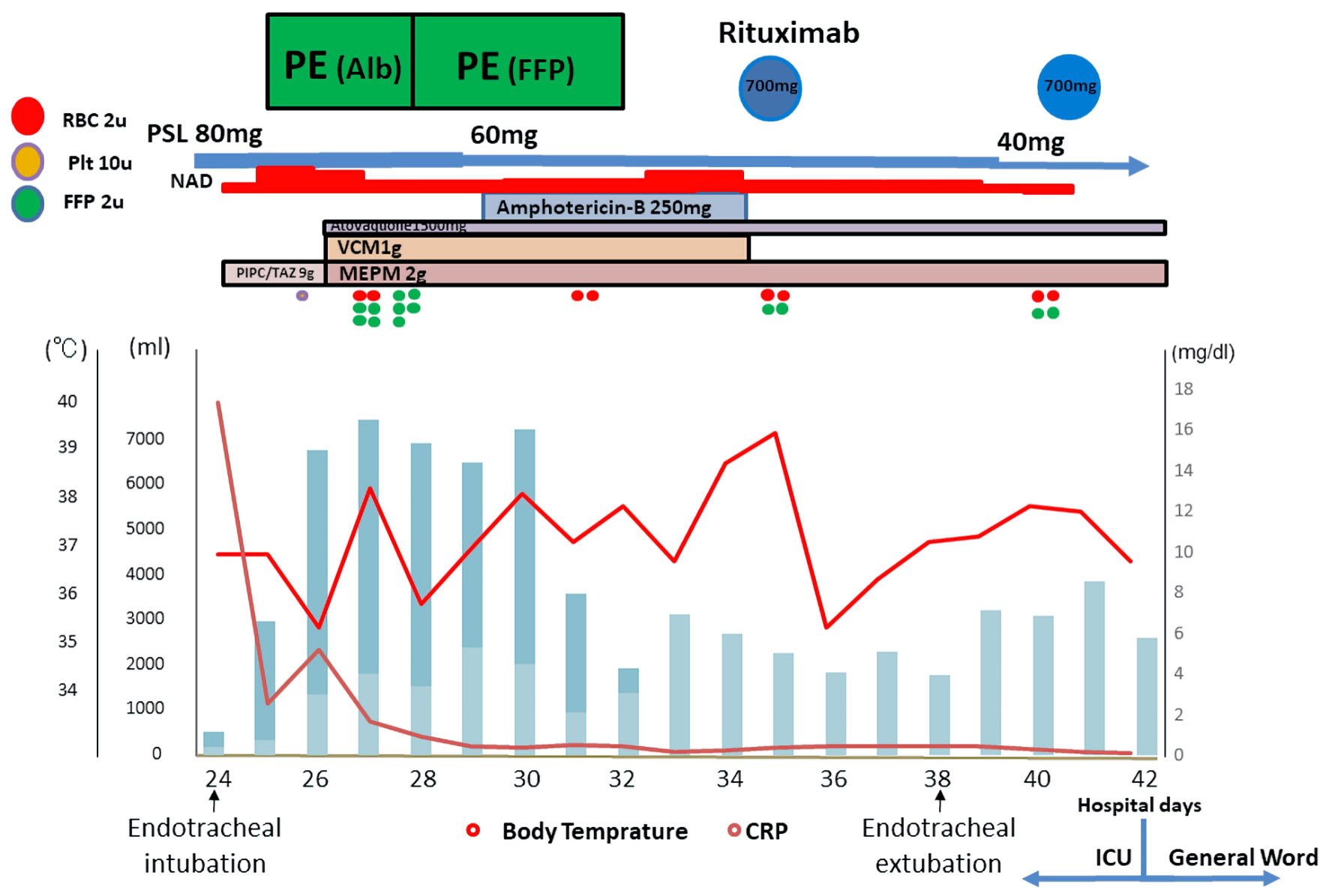 Click for large image | Figure 2. Time course of treatment in the ICU. Mechanical ventilation support was required after endotracheal intubation due to respiratory failure, and CRRT was performed due to renal failure. PE was performed using albumin replacement for 2 days and fibrinogen replacement for 4 days. The rapid initiation of PE was very effective. Her fever quickly declined, her inflammatory reaction decreased, and her anasarca with fluid retention showed a dramatic improvement. After the beneficial effects of PE, rituximab was initiated. PE: plasma exchange; CRRT; continuous renal replacement therapy; RBC: red blood cell; Plt: platelet; FFP: fresh frozen plasma; PSL: prednisolone; NAD: noradrenaline; VCM: vancomycin; PIPC/TAZ: tazobactam/piperacillin; MEPM: meropenem; CRP: C-reactive protein; ICU: intensive care unit. |
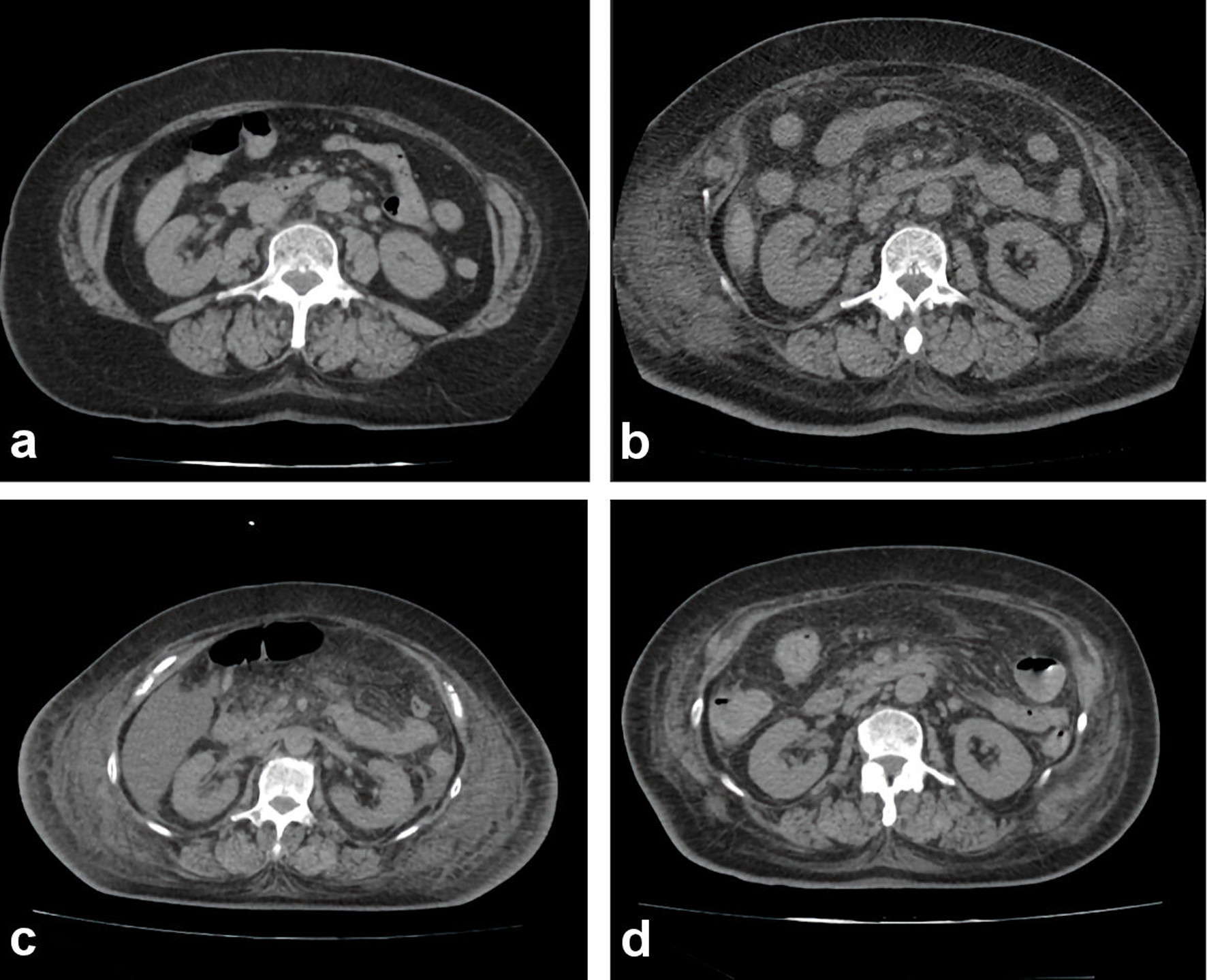 Click for large image | Figure 3. Abdominal computed tomography scan during hospitalization. (a) On admission. (b) Fourteen days after admission. (c) Twenty-four days after admission. (d) Thirty-three days after admission. Anasarca with fluid retention showed extreme progression before ICU admission (until 24th day after admission). After the treatment of plasma exchange, her anasarca with fluid retention showed a dramatic improvement. ICU: intensive care unit; PE: plasma exchange. |
| Discussion | ▴Top |
The diagnostic criteria for TAFRO syndrome were advocated in 2015 [4], and they were updated in 2019 [6]. For the diagnosis of TAFRO syndrome, patients are required to satisfy the three major diagnostic criteria (anasarca with fluid retention, thrombocytopenia, and fever with high inflammatory marker levels) and two minor diagnostic criteria (multiple lymphadenopathy and renal dysfunction) [4]. For the diagnosis of TAFRO syndrome, diseases to be excluded were listed in diagnostic criteria. In present case, to exclude autoimmune disorders, rheumatoid factor, anti-nuclear antibody, MPO-ANCA (P-ANCA), PR3-ANCA (C-ANCA) and other disease-specific autoantibodies were examined. Exclusion of mycobacterial infection such as tuberculosis is also important; thus, examination of interferon-gamma release assays (T-SPOT®), severe fever with thrombocytopenia syndrome (SFTS) virus and tsutsugamushi disease are performed. Polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes (POEMS) syndrome was excluded because the VEGF value was not extremely high. Although the value of macrophage galactose-specific lectin-2 binding protein glycosylation isomer (M2BPGi) was elevated, liver cirrhosis was not revealed by CT scan and other laboratory/physical findings. Signs of previous infection were not observed and ADAMTS13 were not elevated; that is why thrombotic thrombocytopenic purpura (TTP) and hemolytic-uremic syndrome (HUS) were excluded. Meanwhile, lymph node biopsy was hesitated by the following reasons and SS-A and SS-B antibody were not evaluated. All diseases were not completely excluded. It might be one of the limitations of present case report. Since pathology of lymph node revealed the form of MCD in some cases and it is also important to rule out malignant diseases such as malignant lymphoma, the pathological examination of a lymph node biopsy specimen is usually recommended. Meanwhile, in TAFRO syndrome lymph node biopsy is a high-risk procedure due to the presence of severe anasarca and thrombocytopenia. In some cases, the condition is rapidly exacerbated before essential diagnostic assessments can be performed. Indeed, the present case progressed very quickly, and the patient already satisfied the three major diagnostic criteria (anasarca with fluid retention, thrombocytopenia, fever with high inflammatory marker levels) and two minor diagnostic criteria (multiple lymphadenopathy and renal dysfunction) when she was admitted to the ICU. We therefore did not perform lymph node biopsy. In addition, it was difficult to perform the lymph node biopsy due to anasarca, bleeding tendency and unclear of the target lymph node from body surface. However, when we retrospectively reconsider the case, we thought it should be tried to perform lymph node biopsy during “relatively stable ill” period in general word by bringing the platelet up by transfusion. We will make use of this experience next time.
There is no definitive treatment strategy for TAFRO syndrome. According to case reports and experience, treatment is decided on a case-by-case basis [7, 8]. The majority of cases are treated with steroid pulse therapy followed by steroid maintenance therapy and immunosuppressive therapy (e.g., cyclosporin A, tocilizumab, or rituximab) [9]. PE, cyclophosphamide, cyclophosphamide-doxorubicin hydrochloride-oncovin-prednisolone (CHOP), thalidomide, and lenalidomide have been reported to be effective in some refractory cases [7, 8]. Steroid pulse therapy followed by steroid maintenance therapy was already performed in the general ward in the present case in order to stop the disease progression. However, it was invalid, and the patient’s multiple organ failure (e.g., kidney, liver and respiratory failure) due to anasarca was exacerbated. When TAFRO syndrome is resistant to steroid therapy, immunosuppressive agents are often selected as the second-line therapy. The deterioration of her general condition was super acute and potentially fatal; thus, the decision regarding the next treatment was urgent. Although immunosuppressive therapy (e.g., cyclophosphamide, tocilizumab and rituximab) is recognized as the second-line therapy after steroid pulse treatment, PE was performed before immunosuppressive therapy for the following reasons. First, since she was positive for HBs/HBc antibodies, real-time nucleic acid quantitative polymerase chain reaction (PCR) should be performed before immunosuppressive therapy. Second, since she suffered from bacterial infection due to aspiration pneumonia at the time of admission to the ICU, we considered that aggressive immunosuppressive therapy should be avoided. Finally, the performance of PE after immunosuppressive therapy might diminish the medicinal effect due to plasma replacement. We therefore decided to perform PE. Potential root caused by PE was as follows; PE could remove unknown pathogenic substances including cytokines (e.g., IL-6, VEGF), antigen-complexes and antibodies which were associated with the severity of TAFRO syndrome. PE could replace the plasma by albumin solution, which contributed the removal of body flood after adjusting the intravascular volume by oncotic flood. PE is sometimes selected for the treatment of MCD [10], and it might be an effective treatment for TAFRO syndrome that shows resistance to steroid pulse therapy and immunosuppressive agents [5]. The etiology of TAFRO syndrome remains to be elucidated. It is hypothesized that cytokine storm (e.g., IL-6) occurs in this disease due to immunodeficiency. PE might suppress the disease progression by removing cytokines. PE was performed for 7 days in the present case, and we chose albumin as the replacement solution from the start of treatment on the second day. The patient’s condition was not complicated by coagulation abnormality, and she was considered to show intravascular dehydration, despite the presence of severe anasarca; thus, we chose albumin as the replacement solution from the start of treatment on the second day, in order to remove body flood after adjusting the intravascular volume by oncotic flood. We changed the replacement solution to fresh frozen plasma (FFP) on the next 5 days because her fibrinogen level was decreasing after 2 days of PE. The amount of the replacement solution was calculated from the circulation plasma volume. A plasma volume that 1.3 times the circulation plasma volume was determined based on her body weight. We also used CRRT in combination with PE to remove body flood simultaneously. In the process of PE, her fever improved, and then her inflammatory marker levels improved. Her renal function improved and her urinary volume increased without CRRT. Although steroid pulse therapy was not successful in this case, PE contributed to the dramatic improvement of her general condition.
The disease activity was not completely suppressed, even after PE. When she was transferred to the ICU, her severity classification was grade 4, and this recovered to grade 3 after PE. The effect of PE was dramatic; however, additional treatment was required to completely suppress the disease activity. After a negative real-time nucleic acid quantitative PCR result, we decided to administer rituximab. In addition to the PCR result, we had another reason to delay the administration of the rituximab. Indeed, hemodynamic disruption was reported in a case of TAFRO syndrome that was treated with tocilizumab followed by PE [11]. In this case, the patient’s hemodynamics temporarily improved after the initiation of tocilizumab, but collapsed after PE. Finally, the intravenous administration of vasopressor was required. PE has been reported to remove tocilizumab and reduce the blood concentration of immunosuppressive agents. We were therefore concerned that performing PE after treatment with immunosuppressive agents might decrease the immunosuppressive effect. The rate of removal of tocilizumab by conventional apheresis therapies is reported to be approximately 75% [12]. Rituximab treatment was performed for 1 month (four times every 1 week), after which her grade classification improved to grade 1. Based on the present case, we suggest that PE followed by rituximab could be an effective treatment for TAFRO syndrome.
Conclusions
Steroid-resistant TAFRO syndrome is sometimes acutely exacerbated and follows a potentially fatal course. The decision regarding the next treatment, including PE, is urgent in this situation. PE is considered to remove immunosuppressive agents, and reduce their blood concentrations. Although immunosuppressive agents are often selected as second-line therapy after corticosteroids, the performance of PE before treatment with immunosuppressive agents might be an option for steroid-resistant TAFRO syndrome.
Acknowledgments
We are grateful to the staff of the ICU and Cardiovascular Center at Chiba Hokusoh Hospital, Nippon Medical School.
Financial Disclosure
None to declare.
Conflict Interest
None to declare.
Informed Consent
Informed consent for publication was obtained from the patient.
Author Contributions
Yusuke Otsuka, Akihiro Shirakabe, Hirotake Okazaki, Yusaku Shibata, Shota Shigihara and Tomofumi Sawatani treated the patient at ICU. Toshio Asayama treated the patient at general ward. Norio Yokose MD was supervisor at general word of hematology and Kuniya Asai was supervisor at ICU, and both of them supervised the each organization.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Takai K, Nikkuni K, Shibuya H, Hashidate H. [Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly]. Rinsho Ketsueki. 2010;51(5):320-325.
- Fujimoto S, Sakai T, Kawabata H, Kurose N, Yamada S, Takai K, Aoki S, et al. Is TAFRO syndrome a subtype of idiopathic multicentric Castleman disease? Am J Hematol. 2019;94(9):975-983.
doi pubmed - Nishimura Y, Fajgenbaum DC, Pierson SK, Iwaki N, Nishikori A, Kawano M, Nakamura N, et al. Validated international definition of the thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly clinical subtype (TAFRO) of idiopathic multicentric Castleman disease. Am J Hematol. 2021;96(10):1241-1252.
doi pubmed - Masaki Y, Kawabata H, Takai K, Kojima M, Tsukamoto N, Ishigaki Y, Kurose N, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol. 2016;103(6):686-692.
doi pubmed - Meguri Y, Asada N, Nakasako Y, Kondo E, Kambara Y, Yamamoto A, Masunari T, et al. A case report of TAFRO syndrome successfully treated by immunosuppressive therapies with plasma exchange. Ann Hematol. 2019;98(2):537-539.
doi pubmed - Masaki Y, Kawabata H, Takai K, Tsukamoto N, Fujimoto S, Ishigaki Y, Kurose N, et al. 2019 Updated diagnostic criteria and disease severity classification for TAFRO syndrome. Int J Hematol. 2020;111(1):155-158.
doi pubmed - Coutier F, Meaux Ruault N, Crepin T, Bouiller K, Gil H, Humbert S, Bedgedjian I, et al. A comparison of TAFRO syndrome between Japanese and non-Japanese cases: a case report and literature review. Ann Hematol. 2018;97(3):401-407.
doi pubmed - Zhang Y, Suo SS, Yang HJ, Zhou XP, You LS, Yu WJ, Wang ZM, et al. Clinical features and treatment of 7 Chinese TAFRO syndromes from 96 de novo Castleman diseases: a 10-year retrospective study. J Cancer Res Clin Oncol. 2020;146(2):357-365.
doi pubmed - Iwaki N, Fajgenbaum DC, Nabel CS, Gion Y, Kondo E, Kawano M, Masunari T, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91(2):220-226.
doi pubmed - Taniguchi K, Shimazaki C, Fujimoto Y, Shimura K, Uchiyama H, Matsumoto Y, Kuroda J, et al. Tocilizumab is effective for pulmonary hypertension associated with multicentric Castleman's disease. Int J Hematol. 2009;90(1):99-102.
doi pubmed - Lundgren G, Asaba H, Bergstrom J, Groth CG, Magnusson G, Moller E, Strindberg J, et al. Fulminating anti-A autoimmune hemolysis with anuria in a renal transplant recipient: a therapeutic role of plasma exchange. Clin Nephrol. 1981;16(4):211-214.
- Ward DM. Conventional apheresis therapies: a review. J Clin Apher. 2011;26(5):230-238.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


