| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 10, October 2022, pages 521-524
Iatrogenic Immunodeficiency Associated Lymphoproliferative Disorder in a Patient With Inflammatory Bowel Disease
Benjamin D. Germana, Jennifer Akinb, Seo-Hyun Kimc, Caitlin Murphyc, Parameswaran Venugopalc, Nicolas Lopez-Hisijosd, Deborah A. Katzc, e
aDepartment of Internal Medicine, Rush University Medical Center, Chicago, IL, USA
bRush University Medical College, Chicago, IL, USA
cDivision of Hematology, Oncology and Cellular Therapy, Rush University Medical Center, Chicago, IL, USA
dDepartment of Pathology, Rush University Medical Center, Chicago, IL, USA
eCorresponding Author: Deborah Katz, Division of Hematology/Oncology, Department of Internal Medicine, Rush University Medical Center, Chicago, IL 60612, USA
Manuscript submitted January 31, 2022, accepted August 17, 2022, published online October 31, 2022
Short title: IILPD in a Patient With IBD
doi: https://doi.org/10.14740/jmc3798
| Abstract | ▴Top |
Primary colorectal lymphoma is incredibly rare and cases of iatrogenic immunodeficiency associated lymphoproliferative disorder (IILPD) isolated to colorectal area are even more uncommon. Immunodeficiency associated lymphoproliferative disorders can occur in association with primary immune disorders such as inflammatory bowel diseases (IBDs) which are often treated with various immunomodulatory drugs. Of the immunomodulatory drugs, thiopurines, in particular, are known to have a significantly increased relative risk for development of IILPDs. Here we present the case of a 43-year-old Caucasian man with a 22-year history of IBD treated with longstanding immunomodulatory therapy who presented with severe rectal pain and drainage. He underwent an examination under anesthesia with rigid proctoscopy and biopsies were taken of a hard exophytic appearing tissue along the posterior wall of the rectosigmoid junction. Pathological investigation of the samples revealed IILPD. He underwent treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) and achieved complete remission. Literature demonstrates that the use of immunomodulators such as azathioprine has been shown to significantly improve the quality of life in patients with IBD. However, while the absolute risk of lymphoma for any given patient remains quite low, the relative risk of lymphoma in patients who are actively treated with thiopurines is moderate. Therefore, the decision to proceed with thiopurine treatment, especially in the setting of long-term therapy, requires extensive discussion and patient education of the risks/benefits along with closer monitoring of new or uncharacteristic symptoms.
Keywords: Iatrogenic immunodeficiency associated lymphoproliferative disorder; Colorectal lymphoma; Inflammatory bowel disease
| Introduction | ▴Top |
Lymphoproliferative disorders are innate disorders of the immune system in which a population of lymphocytes exhibit disordered growth patterns. Immunodeficiency associated lymphoproliferative disorders refer to an abnormal proliferation of lymphocytes in patients with underlying immunosuppression and are particularly interesting because they form a model in which to explore the relationship between the immune system and the development of malignancy. These disorders are clinically and pathologically heterogeneous, and typically differ based on the underlying setting in which they arise. Immunodeficiency associated lymphoproliferative disorders can occur in association with primary immune disorders or human immunodeficiency virus (HIV) infections. Additionally, they can occur in the setting of iatrogenic immunodeficiency, either in the post-transplant setting after solid organ, stem cell, and bone marrow transplantation or less commonly, during treatment of various autoimmune and rheumatological disorders managed with immunomodulatory agents [1].
Inflammatory bowel diseases (IBDs) are a group of chronic intestinal diseases characterized by inflammation of the bowel. IBD is often treated with various immunomodulatory drugs including thiopurines (TPs), mycophenolate mofetil, methotrexate, and tumor necrosis factor (TNF)-α inhibitors. Thiopurines, in particular, are known to have a significantly increased relative risk for development of iatrogenic immunodeficiency associated lymphoproliferative disorders (IILPDs) [2-4]. Here we present a case of IILPD involving the rectosigmoid junction in a patient with IBD treated with immunomodulatory medications, along with a review of the available literature of this rare condition.
| Case Report | ▴Top |
Investigations
A 43-year-old Caucasian man presented to his gastroenterologist with progressive rectal pain and fevers. His medical history was significant for a 22-year history of ulcerative colitis, which was subsequently diagnosed as Crohn’s disease and iron-deficiency anemia. Following an IBD flare 9 months prior to lymphoma diagnosis, oral steroids were added to his longstanding mesalamine and azathioprine therapy. After 5 months of persistent symptoms, he underwent a flexible sigmoidoscopy which revealed severe colitis. In light of these findings, treatment with vedolizumab was initiated. After two doses, he reported worsening rectal pain and fevers with imaging findings of a perirectal abscess. He underwent abscess drainage which was complicated by colonic perforation requiring emergent Hartmann’s procedure with diverting colostomy and seton drain placement. Pathology at that time was without evidence of lymphoma.
The patient presented to our institution a few months later for a second opinion given ongoing severe rectal pain and drainage. He underwent an examination under anesthesia with rigid proctoscopy. Digital examination was notable for hard, neoplastic feeling tissue along the posterior wall of the rectum and biopsies were taken of a hard exophytic appearing tissue (Fig. 1).
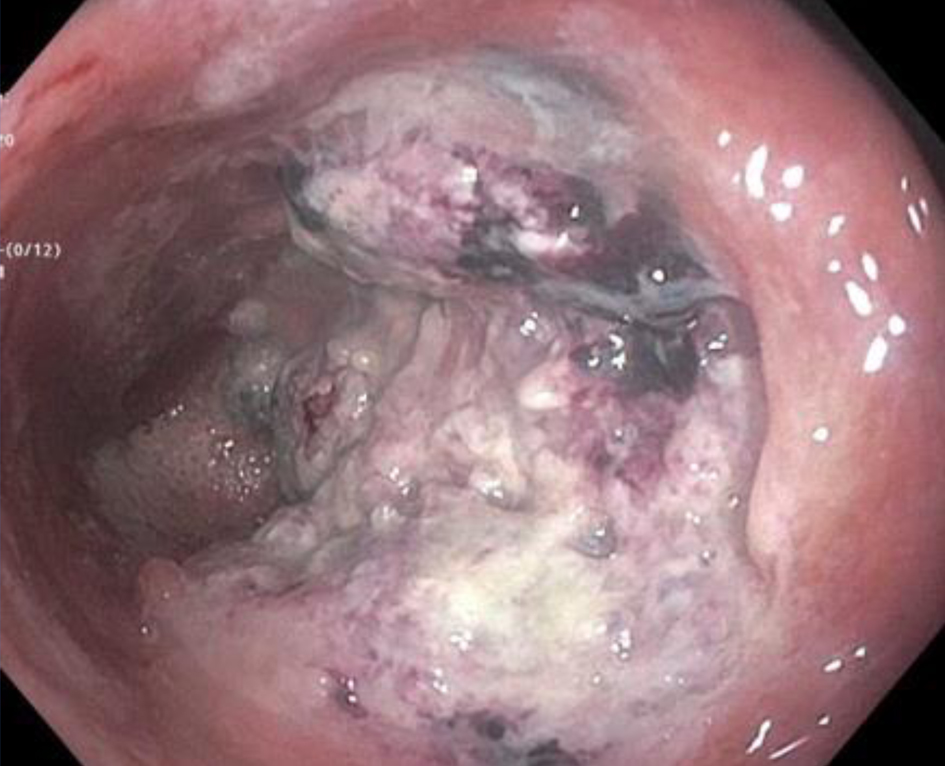 Click for large image | Figure 1. Endoscopic view of an exophytic mass on posterior rectal wall. |
Diagnosis
He subsequently underwent a colonoscopy and endoscopy with biopsies. The pathology from rectal biopsies revealed IILPD, monomorphic, diffuse large B-cell lymphoma, non-germinal center (Fig. 2). Fluorescence in situ hybridization (FISH) studies were negative for MYC, BCL-2 and BCL-6 rearrangements. Other biopsied sites were without evidence of lymphoma. Mesalamine and azathioprine were discontinued, and patient was referred to hematology for further management.
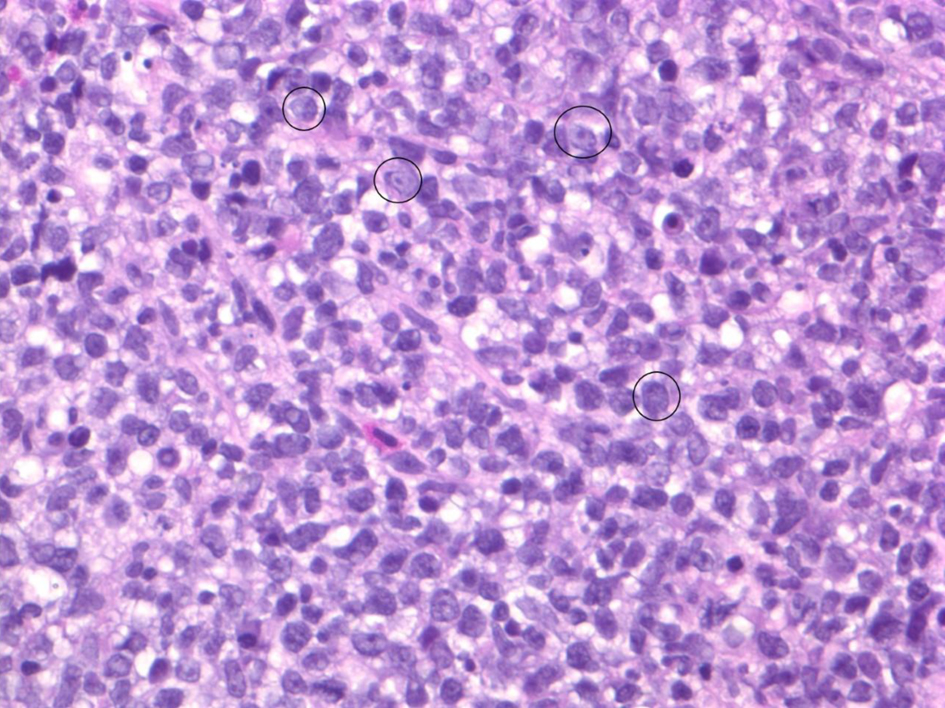 Click for large image | Figure 2. Biopsy of exophytic rectal mass demonstrating characteristic morphology of diffuse B-cell lymphoma: diffuse infiltrate of large atypical B cells with brisk mitotic activity. Note the majority of cells seen are neoplastic B cells. A few particularly large B cells are circled in black. |
Treatment and follow-up
Staging studies including computed tomography (CT) scan, whole body positron emission tomography (PET) scan and bone marrow biopsy/aspirate revealed disease localized to the rectum. The patient has since completed six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP), and achieved complete response which was confirmed by post-treatment PET scan as well as colonoscopy with random biopsies.
| Discussion | ▴Top |
Primary colorectal lymphoma (PCL) is an uncommon colonic malignancy comprising only 0.2% to 0.6% of all large bowel malignancies [5]. PCL is an uncommon site of extra-nodal non-Hodgkin lymphoma (NHL), and accounts for approximately 10-20% of gastrointestinal NHL [6-8]. PCL more frequently involves the cecum compared to the rectum, likely due to differences in the amount of lymphoid tissue in these two regions [9-12].
Epidemiological studies show that the incidence of colorectal lymphomas is highest between the ages of 50 and 70 years, with a 2:1 male predominance [13]. The majority of patients with PCL present with early stage disease (stage IE) [14]. Diffuse large B-cell lymphoma (DLBCL) is the most common histological subtype occurring in the colon and rectum [5, 8, 15, 16]. Several studies have reported a small, but significant increased risk of lymphoma associated with use of thiopurines [2, 4, 17, 18]. Kotlyar and colleagues published a meta-analysis where they estimated a standardized incidence rate of thiopurine associated lymphoma of 2.80 (95% confidence interval (CI), 1.82 - 4.32) in population-based studies [2]. These authors demonstrated that patients with IBD who were taking thiopurines had nearly a six-fold higher incidence of lymphoma when compared with the general population. Similar to other studies, this meta-analysis demonstrated that the magnitude of risk is associated with duration of treatment with increased risk of lymphoma in those with more than 1 year of active treatment. As in other studies, findings from this meta-analysis suggest that the increased incidence of lymphoma does not persist after treatment with thiopurines is discontinued [2, 3]. This suggests that the driving factor in thiopurine associated lymphoma is via immunosuppression rather than direct DNA damage.
Due to its rarity, there is no standard of care or high-level evidence to direct management of PCLs. Many experts suggest a multidisciplinary approach to treatment with involvement of surgical oncology, gastroenterology, hematology, and radiation oncology. Additionally, treatment decisions should factor in presenting symptoms (e.g., obstruction, bleeding, perforation), intent of treatment (curative versus palliative) and histological sub-type of NHL given variable responses to chemotherapy.
The role for surgery in the upfront management of PCL remains unclear despite high rates of surgical intervention in patients. Surgery is often used as a diagnostic tool when there is an initial suspicion for carcinoma. Additionally, patients may present with acute concerns such as bleeding, perforation, or obstruction that require urgent or emergent surgical intervention.
There are no randomized controlled studies comparing surgery plus chemotherapy with chemotherapy alone. Some advocate for surgical intervention for localized disease involving large lesions, particularly those localized to the colon, in the hope that this will decrease rates of perforation and bleeding, minimize chemotherapy-related toxicity and potentially improve survival outcomes [13, 19, 20]. The benefit of surgical resection followed by chemotherapy versus chemotherapy alone has been assessed in several nonrandomized studies, and has demonstrated superior survival rates in those treated with surgery followed by chemotherapy versus chemotherapy alone [21-24]. One large retrospective analysis of patients with limited stage intestinal DLBCL demonstrated lower recurrence rate (15.3% versus 36.8%) and improved 3-year overall survival rate (91% versus 62%) in patients treated with surgery plus chemotherapy versus those treated with chemotherapy alone [21]. Another prospective nonrandomized study which included patients with limited stage gastrointestinal DLBCL also demonstrated favorable outcomes in those patients treated with primary surgical resection followed by chemotherapy [22]. A Surveillance, Epidemiology and End Results (SEER) database study additionally demonstrated better overall survival for patients with PCL treated with surgery compared to those treated with chemotherapy alone. However, a subgroup analysis demonstrated that certain groups including those with rectal disease, left-sided disease, and certain histological subtypes did not show a survival benefit from surgery [19]. The authors acknowledged that there is a lack of evidence to support a correlation of surgical intervention with improved survival. The study was limited by lack of important clinical information including chemotherapy data, selection bias, and variations in data reporting and coding systems [19]. Hangge and colleagues report on their experience of PCL by comparing an institutional cohort to SEER database. While institutional patients who underwent surgery had the highest 5-year overall survival, these authors concluded that treatment modality of surgery versus no surgery was not predictive of overall survival [5]. In fact, the most common treatment in the institutional cohort was chemotherapy alone. The higher survival rate in surgical patients was thought to be impacted by preoperative selection bias, disease stage, and histological subtype. Additionally, a subgroup analysis did not detect any significant differences; however, small sample sizes limited the ability to power for this analysis [5]. Another systematic review of primary gastrointestinal NHL of the small and large intestines additionally concluded survival benefit in those treated with surgery [16]. However, the authors note several limitations that prevent “evidence-based generalizable conclusions about optimal treatment practices” [16]. Some of the limitations noted included selection bias in operative candidates, aggressive histological subtypes necessitating emergent surgeries and pre-rituximab era medical management not reflecting optimal outcomes.
Many experts argue that chemotherapy alone should remain the standard therapy for patients with a highly proliferative aggressive lymphoma given high response rate and potential for cure with R-CHOP-like regimens. Additionally, the infiltrative and systemic nature of lymphoma often precludes it from being managed with surgical resection or for extent of disease to be encompassed in radiation fields [19, 25]. Skubbe and colleagues conducted a retrospective review of patients with colorectal lymphoma, and did not demonstrate a difference in overall survival between those treated with surgical management versus chemotherapy alone [14].
Learning points
PCL is an uncommon disease, and cases of IILPD with isolated involvement of the colorectal area are even rarer. While the relative risk of lymphoma in patients who are actively treated with thiopurines is moderate, the absolute risk of lymphoma for any given patient remains quite low. Therefore, the decision to proceed with thiopurine treatment requires risk/benefit discussion. At this time, there is no current standard of care treatment for PCL or IILPD, and management should be tailored to the individual patient using a multidisciplinary approach.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Per our academic center institutional guidelines regarding case reports, no IRB application was required nor was specific informed consent needed for case report.
Author Contributions
Authors Benjamin German and Deborah Katz were primarily contributors to the manuscript including case presentation and review of literature. Authors Seo-Hyun Kim, Caitlin Murphy, Jennifer Akin and Parameswaran Venugopal assisted with input/feedback on manuscript and review of literature. Author Nicolas Lopez-Hisijos provided input regarding pathological/histological aspects to this case/manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Swerdlow S, Craig F. Iatrogenic Immunodeficiency-Associated Lymphoproliferative Disorders. Hematopathology, Chapter 55. 1013-1029.e8.
- Kotlyar DS, Lewis JD, Beaugerie L, Tierney A, Brensinger CM, Gisbert JP, Loftus EV, Jr., et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015;13(5):847-858.e4.
doi pubmed - Khan N, Abbas AM, Lichtenstein GR, Loftus EV, Jr., Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145(5):1007-1015.e1003.
doi pubmed - Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lemann M, Cosnes J, Hebuterne X, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617-1625.
doi - Hangge PT, Calderon E, Habermann EB, Glasgow AE, Mishra N. Primary Colorectal Lymphoma: Institutional Experience and Review of a National Database. Dis Colon Rectum. 2019;62(10):1167-1176.
doi pubmed - Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin's lymphoma in a population-based registry. Br J Cancer. 1999;79(11-12):1929-1934.
doi pubmed - Andrews CN, John Gill M, Urbanski SJ, Stewart D, Perini R, Beck P. Changing epidemiology and risk factors for gastrointestinal non-Hodgkin's lymphoma in a North American population: population-based study. Am J Gastroenterol. 2008;103(7):1762-1769.
doi pubmed - Cheung MC, Housri N, Ogilvie MP, Sola JE, Koniaris LG. Surgery does not adversely affect survival in primary gastrointestinal lymphoma. J Surg Oncol. 2009;100(1):59-64.
doi pubmed - Stanojevic GZ, Stojanovic MP, Stojanovic MM, Krivokapic Z, Jovanovic MM, Katic VV, Jeremic MM, et al. Non-Hodgkin's lymphomas of the large bowel-clinical characteristics, prognostic factors and survival. Acta Chir Iugosl. 2008;55(3):109-114.
doi pubmed - Fan CW, Changchien CR, Wang JY, Chen JS, Hsu KC, Tang R, Chiang JM. Primary colorectal lymphoma. Dis Colon Rectum. 2000;43(9):1277-1282.
doi pubmed - Gonzalez QH, Heslin MJ, Davila-Cervantes A, Alvarez-Tostado J, de los Monteros AE, Shore G, Vickers SM. Primary colonic lymphoma. Am Surg. 2008;74(3):214-216.
doi pubmed - Wong MT, Eu KW. Primary colorectal lymphomas. Colorectal Dis. 2006;8(7):586-591.
doi pubmed - Gay ND, Chen A, Okada CY. Colorectal lymphoma: a review. Clin Colon Rectal Surg. 2018;31(5):309-316.
doi pubmed - Skube SJ, Arsoniadis EG, Sulciner ML, Gilles SR, Gaertner WB, Madoff RD, Melton GB, et al. Colorectal lymphoma: a contemporary case series. Dis Colon Rectum. 2019;62(6):694-702.
doi pubmed - Cai YB, Chen HY, He JJ, Hu YT, Yang Q, Chen LB, Xiao Q, et al. The role of surgical intervention in primary colorectal lymphoma: A SEER population-based analysis. Oncotarget. 2016;7(44):72263-72275.
doi pubmed - Lightner AL, Shannon E, Gibbons MM, Russell MM. Primary Gastrointestinal Non-Hodgkin's Lymphoma of the Small and Large Intestines: a Systematic Review. J Gastrointest Surg. 2016;20(4):827-839.
doi pubmed - Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679-1686.
doi pubmed - Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54(8):1121-1125.
doi pubmed - Cai S, Cannizzo F, Jr., Bullard Dunn KM, Gibbs JF, Czuczman M, Rajput A. The role of surgical intervention in non-Hodgkin's lymphoma of the colon and rectum. Am J Surg. 2007;193(3):409-412; discussion 412.
doi pubmed - Vaidya R, Habermann TM, Donohue JH, Ristow KM, Maurer MJ, Macon WR, Colgan JP, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24(9):2439-2443.
doi pubmed - Kim SJ, Kang HJ, Kim JS, Oh SY, Choi CW, Lee SI, Won JH, et al. Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood. 2011;117(6):1958-1965.
doi pubmed - Lee J, Kim WS, Kim K, Ahn JS, Jung CW, Lim HY, Kang WK, et al. Prospective clinical study of surgical resection followed by CHOP in localized intestinal diffuse large B cell lymphoma. Leuk Res. 2007;31(3):359-364.
doi pubmed - Bellesi G, Alterini R, Messori A, Bosi A, Bernardi F, di Lollo S, Ferrini PR. Combined surgery and chemotherapy for the treatment of primary gastrointestinal intermediate- or high-grade non-Hodgkin's lymphomas. Br J Cancer. 1989;60(2):244-248.
doi pubmed - Koh PK, Horsman JM, Radstone CR, Hancock H, Goepel JR, Hancock BW. Localised extranodal non-Hodgkin's lymphoma of the gastrointestinal tract: Sheffield Lymphoma Group experience (1989-1998). Int J Oncol. 2001;18(4):743-748.
doi pubmed - Koniaris LG, Drugas G, Katzman PJ, Salloum R. Management of gastrointestinal lymphoma. J Am Coll Surg. 2003;197(1):127-141.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


