| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 1, January 2022, pages 26-30
Immunoglobin A Deficiency and Squamous Cell Carcinoma With a Rare Presentation as Anal Cancer
Anup Kasia, g, Stijn Hentzenb , Nikhil Guptaa, Padma Poddutooria, Rashna Madanc, Shrikant Anantd, Sufi Mary Thomasd, e, f
aDepartment of Oncology, The University of Kansas Medical Center, Kansas City, KS, USA
bDepartment of Internal Medicine, The University of Kansas Medical Center, Kansas City, KS, USA
cDepartment of Pathology and Laboratory Medicine, The University of Kansas Medical Center, Kansas City, KS, USA
dDepartment of Cancer Biology, The University of Kansas Medical Center, Kansas City, KS, USA
eDepartment of Otolaryngology, The University of Kansas Medical Center, Kansas City, KS, USA
fDepartment of Anatomy and Cell Biology, The University of Kansas Medical Center, Kansas City, KS, USA
gCorresponding Author: Anup Kasi, Department of Oncology, The University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA
Manuscript submitted October 4, 2021, accepted November 17, 2021, published online January 17, 2022
Short title: IgA Deficiency and Anal SCC
doi: https://doi.org/10.14740/jmc3804
| Abstract | ▴Top |
Selective immunoglobin A deficiency (IgAD) is the most common immunodeficiency disorder in the western world. Cancer is the most common cause of death in these individuals. Various cases have been reported of squamous cell carcinoma (SCC) in IgAD at sites like skin, oral cavity, and lung. Here we present a rare case of SCC occurring as anal cancer. No other reports to our knowledge describe this rare presentation. A 54-year-old Caucasian woman with asymptomatic partial IgAD presented with a palpable anal mass. Further evaluation showed stage IIIa SCC anal cancer (T1N1M0). Additional workup showed positive human papilloma virus (HPV) serology and positive HPV immunohistochemistry studies. The patient achieved complete response with chemoradiation with her most recent imaging and anorectal exam showing no evidence of cancer recurrence at 3 years follow-up. This case highlights the association between IgAD and malignancy. Although IgAD is the most common primary antibody deficiency, this patient’s case presents a rare instance of anal SCC in an IgA-deficient individual. Studies show an association between HPV infection and SCC, but few include IgA-deficient individuals. Patients with IgAD and other immunodeficiencies are at higher risk for HPV infection and therefore may be at a higher risk of SCC. With widespread use of the HPV vaccine, the medical community should be aware of its importance in cancer prevention for these patients. Further studies are needed to evaluate relationships between IgAD, HPV infections, SCC cancer, and the role that the HPV vaccine has in cancer prophylaxis.
Keywords: Anal cancer; Gastrointestinal cancer; IgA deficiency; Immunodeficiency; HPV infection; Cancer prevention
| Introduction | ▴Top |
Selective immunoglobin A deficiency (IgAD) is defined in two ways based on serum immunoglobulin levels. Severe deficiency is defined by an IgA level less than 7 mg/dL with normal IgM and IgG levels while partial deficiency is defined by a serum IgA concentration greater than 7 mg/dL but two standard deviations below the patient’s age adjusted mean [1]. Additionally, these patients are defined as having a normal IgG antibody response to vaccinations [2]. Population studies have found it to be the most common immunodeficiency in the western world with an occurrence of 1:400 to 1:3,000 individuals [3]. There is a demonstrated link between IgAD and giardiasis, celiac disease, nodular lymphoid hyperplasia, ulcerative colitis, Crohn’s disease, pernicious anemia, and gastric and colorectal adenocarcinoma [1, 4]. Here we present a rare case of squamous cell carcinoma (SCC) of anal canal in an IgA-deficient individual who also was found to have human papilloma virus (HPV)-positive immunohistochemistry (IHC) staining. An extensive literature search was unable to identify a similar presentation in an IgA-deficient individual. Previous studies have revealed a link between HPV infection and SCC in these individuals [5-7]. However, none of these describe a case of anal SCC. We review the literature on the main clinicopathological aspects of this important condition and try to explore these associations. Lastly, we assess whether these links will help us propose a new treatment or prophylaxis for these cancers.
| Case Report | ▴Top |
Investigations
A 54-year-old Caucasian woman with history of hypothyroidism, migraine, psoriatic arthritis, and Raynaud’s syndrome was incidentally diagnosed with a rectal mass by her gynecologist during an annual pelvic exam. She was largely asymptomatic other than a single episode of hematochezia about 1 year prior to presentation that was attributed to internal hemorrhoids and itching/burning in her rectum with bowel movements. Otherwise, she denied any abdominal pain, appetite change or other bowel/bladder disturbances. Physical examination revealed temperature of 36.7 °C (98 °F), heart rate of 70, respiratory rate of 14 per minute and blood pressure of 115/64 mm Hg. Her exam was remarkable for a rectal mass. Further interviewing of the patient was unremarkable for any immediate family history of cancer.
Diagnosis
After identification of the rectal mass, she then underwent a computed tomography (CT) of abdomen and pelvis which revealed mild eccentric mural thickening of posterolateral distal rectum and an enlarged left perirectal lymph node. This was followed by a colonoscopy that revealed a rectal mass 0.5 cm above the anal verge. Subsequently, a lower endoscopic ultrasound showed a hypoechoic rectal mass 1.7 × 1.7 × 0.5 cm that was 0.5 cm above the anal verge with a malignant appearing lymph node 7 × 8 mm in the left perirectum. A distal rectal biopsy revealed the mass to be a poorly differentiated SCC and fine-needle aspiration cytology proved the lymph node to be malignant. Hence her TNM staging was T1N1M0. A positron emission tomography (PET) scan revealed activity in anal canal with standardized uptake value (SUV) 17.4 and three left perirectal nodes with a maximum SUV of 6.72. Her carcinoembryonic antigen (CEA) level was 0.4 µg/L, which was expected as the tumor was identified as an SCC instead of an adenocarcinoma. Her IgA level was found to be 74 mg/dL (age-based two standard deviation range: 87 - 352 mg/dL) indicating her to be IgA-deficient. Other studies were significant for human immunodeficiency virus (HIV)-negative serology and HPV-positive serology. The microsatellite instability (MSI) panel of the core biopsy of her rectal mass was negative for any loss in nuclear expression of mismatch repair (MMR) proteins, consistent with microsatellite stable (MSS) tumor.
IHC studies showed the patient’s tumor to be HPV-positive (Fig. 1). In addition, the patient’s tumor exhibited high infiltration of T cells, as demonstrated by CD3+ staining, with the majority of these cells being cytotoxic T lymphocytes (Fig. 1). Infiltration of CD4+ helper T cells was relatively sparse in comparison, comparable to low levels of B-cell and macrophage infiltration, as marked by CD20 and CD68 staining, respectively. The tumor was positive for programmed death ligand 1 (PD-L1) expression, indicating tumor infiltrating T cells were likely suppressed.
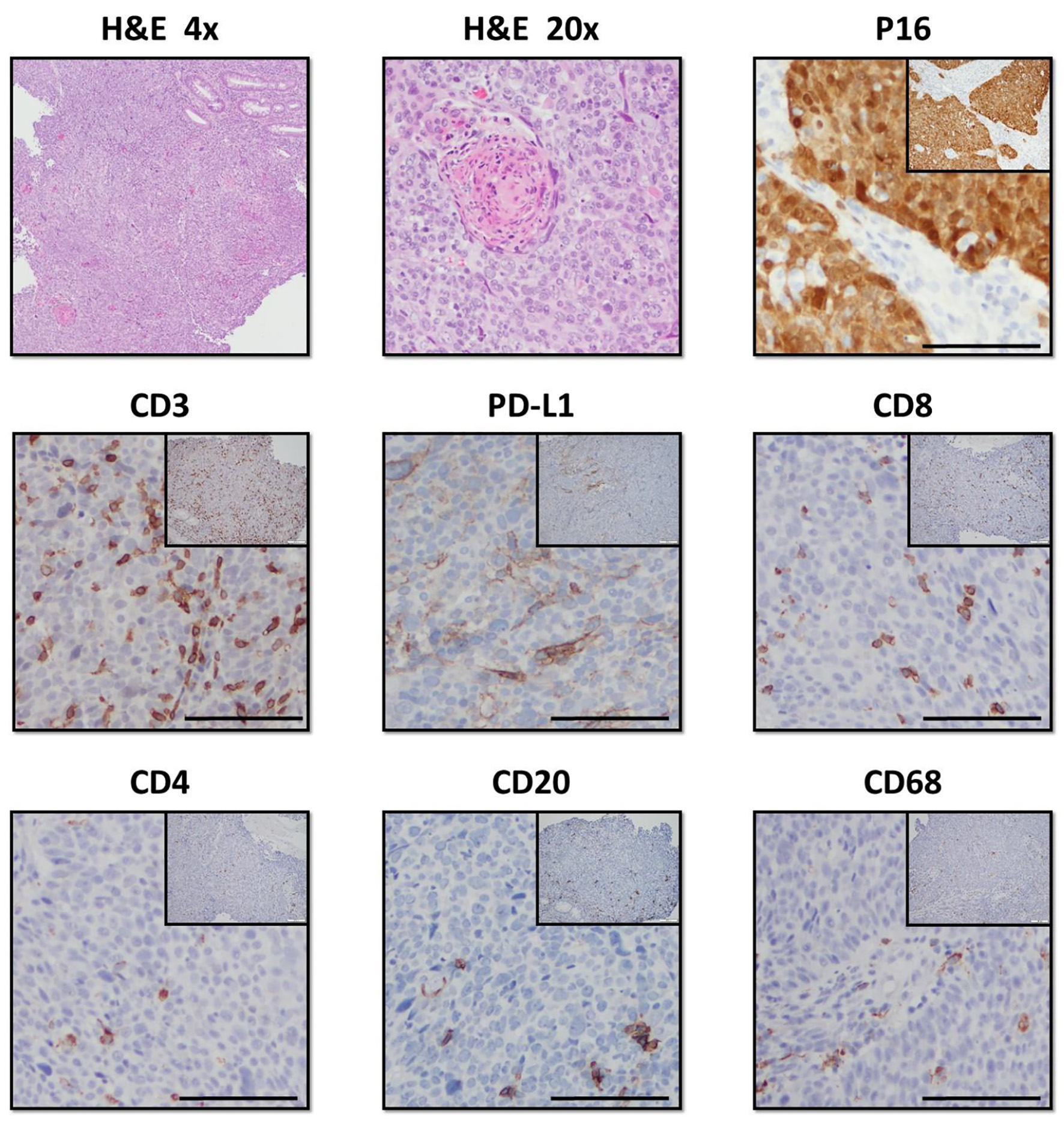 Click for large image | Figure 1. Histologic staining of the patient’s anal SCC tumor. The tumor stained positive for p16 in the squamous epithelium, indicating HPV infection. Hematoxylin and eosin (H&E) staining revealed a high degree of immune infiltration into the tumor. Immunohistochemistry stained positive for CD3, CD8, and CD4, indicating both cytotoxic and helper T cells are present in the tumor’s immune infiltrate. Of note, the tumor also stained positively for PD-L1, a known T-cell inhibitor. The anal SCC also stained positively for CD20 and CD68, revealing the presence of both B cells and monocytes in the infiltrate. SCC: squamous cell carcinoma; HPV: human papilloma virus; PD-L1: programmed death ligand 1. |
Treatment
The patient was started on chemoradiation per Nigro protocol using 5-fluorouracil (FU), mitomycin, and radiation therapy for 5 weeks as recommended by NCCN guidelines. The patient tolerated treatment well without complication and achieved a complete response.
Follow-up and outcomes
Now 3 years post therapy, the patient’s most recent imaging and anorectal exam show no active disease.
| Discussion | ▴Top |
This rare case of SCC in an IgA-deficient individual had many unique aspects. The most remarkable fact is its site, the anal canal, as there is no literature or case report yet reporting anal SCC in an IgA-deficient individual that we could find. This case sheds light on the correlation between IgA deficiency and SCC, of which there have been previous anecdotal reports, but this is the first case being reported to present as anal cancer.
An attempt to understand the pathogenesis of SCC in this immune deficiency state requires us to briefly review the biology of IgA. IgA is the second most common immunoglobulin in human serum after IgG, and is the predominant immunoglobulin found in mucosal secretions. Both serum and secretory IgA are produced en masse by mature plasma cells localized in germinal centers or near mucosal surfaces. While IgA found in the serum is monomeric, mucosal plasma cells secrete IgA as a dimer linked by an additional protein J chain [3]. Here, the IgA dimer binds the polymeric Ig receptor found on the basolateral surface of the epithelium and is transported through the epithelial layer [8, 9]. Once secreted to the apical surface, a portion of the polymeric Ig receptor is proteolytically cleaved from the receptor and covalently bound to the IgA dimer and J chain, rendering the secreted IgA complex resistant to proteolysis [10]. IgAD is a primary immunodeficiency disease presumed to result from a failure of terminal differentiation in IgA-positive B cells. The transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) receptor and two ligands, B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), likely play a role in the pathogenesis of defective humoral immunity. TACI is a tumor necrosis receptor superfamily member that is involved in lymphocyte maturation and survival [11] and in murine B cells, BAFF treatment stimulates IgA production, while TACI deletion completely abrogates this response [12].
Reduced concentrations of IgA cause an increase in the body’s exposure to new antigens that normally would have been neutralized by the IgA at their point of entry [13]. Many of these antigens are carcinogens, such as HPV for example. Previous studies have already established HPV as a causative factor of SCC of cervix and head and neck, but its occurrence in an individual with IgA deficiency and the role this deficiency plays in this cancer is our main interest. Alvarez et al reported two cases highlighting the occurrence of SCC in IgA-deficient individuals with HPV infections [14]. Both were females with vaginal HPV, infection, and SCC of oral cavity. Biopsy of the oral mucosa showed signs of HPV. In addition, there have been studies that have linked HPV with epidermoid cancer of anal canal [5, 15]. Our patient’s case highlights the role IgAD might have in the pathogenesis of anal SCC in the setting of an HPV infection.
Numerous reports in the literature show that immunodeficiency may potentiate the progression from HPV infection to development neoplastic lesions. One report showed a 20-fold increase in the incidence of anal SCC in renal transplant patients taking immunosuppressive medication compared to the general population [16]. Similarly, risk of cervical cancer in HPV+ patients has been shown to be higher in those on immunosuppressive therapy [17]. Our patient’s case serves as further evidence solidifying the link between immunodeficiency in HPV+ patients to an increased incidence of cancer.
There is widespread support for use of the HPV vaccine in reducing the risk of anal cancer [18, 19]. In 2014, the 9-valent HPV virus-like particle vaccine was approved by the US Food and Drug Administration (FDA). More recently, in 2018, the FDA expanded the indication for the 9-valet HPV vaccine to include women and men aged 27 - 45 years old [20]. It has been included in the list of vaccines for routine immunization [21]. Given the evidence in the literature linking HPV infections to cancer and the reports of increased risk of HPV infection shown in immunosuppressed patients, it is clear these patients should receive prophylaxis with the 9-valent HPV vaccine. As there is already widespread use of the HPV vaccine, the medical community should be aware of its importance in IgA-deficient and other immunosuppressed patients. Furthermore, providers should be encouraged to vaccinate adult IgA-deficient patients up to age 45 with the recent expansion of indicated age range by the FDA.
Learning points
This unique case of a patient with IgA deficiency and SCC anal cancer found to have HPV-positive IHC studies emphasizes the relationship between the three pathologies. It has been shown that patients with IgA deficiency have an increased risk of cancer and literature review shows that patients with immunosuppression are at increased risk of HPV infection. This risk of HPV infections puts patients with IgA deficiency at higher risk of SCC cancers as seen in the patient case presented. Given that there is widespread use of the 9-valent HPV vaccine and that the indicated age range has been recently expanded, the medical community should be made aware of its importance in cancer prevention for IgA-deficient individuals as well as other individuals with immunosuppression. Further studies should be pursued to evaluate the relationship between IgA deficiency, HPV infections, SCC cancer, and the role that the HPV vaccine has in cancer prophylaxis for these patients.
Acknowledgments
We acknowledge support from the University of Kansas (KU) Cancer Center’s Biospecimen Repository Core Facility staff for helping obtain human specimens and for performing histological work. We acknowledge Tejashree Karnik for assistance with figures. The authors also acknowledge support from the KU Cancer Center’s Cancer Center Support Grant (P30 CA168524) and donations from The Thomas P. O’Sullivan IV and Marina O’Sullivan Family Fund.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no competing interests.
Informed Consent
Written informed consent was obtained from the patient and the patient’s next of kin for publication of this report and any accompanying images.
Author Contributions
Anup Kasi: care of patient, literature search, and manuscript preparation and editing. Stijn Hentzen and Nikhil Gupta: literature search and manuscript preparation. Padma Poddutoori: care of patient and patient information preparation. Rashna Madan and Shrikant Anant: pathology and diagnostic analysis of patient, and figure preparation. Sufi Mary Thomas: pathology and diagnostic analysis of patient, figure preparation, and manuscript editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CT: computed tomography; CEA: carcinoembryonic antigen; IgAD: immunoglobin A deficiency; HPV: human papilloma virus; HIV: human immunodeficiency virus; TACI: transmembrane activator and calcium modulator and cyclophilin ligand interactor; BAFF: B-cell activating factor; SCC: squamous cell carcinoma
| References | ▴Top |
- Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10-16.
doi pubmed - Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190-197.
doi pubmed - Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol. 2001;21(5):303-309.
doi pubmed - Ludvigsson JF, Neovius M, Ye W, Hammarstrom L. IgA deficiency and risk of cancer: a population-based matched cohort study. J Clin Immunol. 2015;35(2):182-188.
doi pubmed - Gami B, Kubba F, Ziprin P. Human papilloma virus and squamous cell carcinoma of the anus. Clin Med Insights Oncol. 2014;8:113-119.
doi pubmed - Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC, Thompson TD, Razzaghi H, et al. Human papillomavirus-associated cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
doi pubmed - Wentzensen N, Follansbee S, Borgonovo S, Tokugawa D, Schwartz L, Lorey TS, Sahasrabuddhe VV, et al. Human papillomavirus genotyping, human papillomavirus mRNA expression, and p16/Ki-67 cytology to detect anal cancer precursors in HIV-infected MSM. AIDS. 2012;26(17):2185-2192.
doi pubmed - Mostov KE, Friedlander M, Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984;308(5954):37-43.
doi pubmed - Singer KL, Mostov KE. Dimerization of the polymeric immunoglobulin receptor controls its transcytotic trafficking. Mol Biol Cell. 1998;9(4):901-915.
doi pubmed - Fallgreen-Gebauer E, Gebauer W, Bastian A, Kratzin HD, Eiffert H, Zimmermann B, Karas M, et al. The covalent linkage of secretory component to IgA. Structure of sIgA. Biol Chem Hoppe Seyler. 1993;374(11):1023-1028.
doi pubmed - Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, Scott ML, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111(3):1004-1012.
doi pubmed - Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201(1):35-39.
doi pubmed - Ludvigsson JF, Neovius M, Hammarstrom L. Association between IgA deficiency & other autoimmune conditions: a population-based matched cohort study. J Clin Immunol. 2014;34(4):444-451.
doi pubmed - Alvarez J, Garip E, Benitez M, Guzman L. Immunoglobulin a deficiency, HPV and oral cancer. The World Allergy Organization Journal. 2012;5(Suppl 2):S191-S191.
doi - Frisch M, Glimelius B, van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, Goldman S, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337(19):1350-1358.
doi pubmed - Sillman FH, Sentovich S, Shaffer D. Ano-genital neoplasia in renal transplant patients. Ann Transplant. 1997;2(4):59-66.
- Ozsaran AA, Ates T, Dikmen Y, Zeytinoglu A, Terek C, Erhan Y, Ozacar T, et al. Evaluation of the risk of cervical intraepithelial neoplasia and human papilloma virus infection in renal transplant patients receiving immunosuppressive therapy. Eur J Gynaecol Oncol. 1999;20(2):127-130.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr., Penny ME, Aranda C, Vardas E, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401-411.
doi pubmed - Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr., Aranda C, Jessen H, Hillman R, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576-1585.
doi pubmed - Montague L. Summary basis for regulatory action. U.S. Food and Drug Administration. 2018.
- Food and Drug Administration. Highlights of prescribing informations. Gardasil 9 (Human Papillomavirus 9-valent Vaccine, Recombinant). 2015. Retrieved from http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM426457.pdf.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


