| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 5, May 2022, pages 219-224
Acute Hemolytic Anemia Caused by Loxoscelism Treated With Plasmapheresis: A Case Report
Stephanie Harrya, Emily Brugionia, b, Sheshadri Madhusudhanaa
aDepartment of Hematology/Oncology, University of Missouri Kansas City, Kansas City, MO 64108, USA
bCorresponding Author: Emily Brugioni, Department of Hematology/Oncology, University of Missouri Kansas City, Kansas City, MO 64108, USA
Manuscript submitted November 22, 2021, accepted February 7, 2022, published online April 23, 2022
Short title: Acute Hemolytic Anemia Caused by Loxoscelism
doi: https://doi.org/10.14740/jmc3828
| Abstract | ▴Top |
The bite of a brown recluse spider (Loxosceles reclusa) is usually associated with skin necrosis; however, it can lead to more significant morbidity including acute hemolytic anemia, rhabdomyolysis, disseminated intravascular coagulopathy and death. Here we highlight a case using plasmapheresis as treatment for acute hemolytic anemia caused by the bite of a brown recluse spider. A 49-year-old male presented to the emergency room 5 days after suffering a spider bite due to worsening symptoms. He had worsening pain at the site of the bite, diffuse body myalgias, darkening of his urine, chills, and shortness of breath. Hematology was consulted to assist in the management of hemolytic anemia refractory to multiple blood transfusions, worsening acute kidney failure requiring hemodialysis, and concern for impending death. After a literature review suggesting plasmapheresis may be beneficial in this scenario, the case was discussed with the local blood bank, and plasmapheresis was initiated. The patient underwent plasmapheresis with albumin for 2 days and the patient’s hemoglobin improved and stabilized. Therapy of loxoscelism is directed at limiting the dermatonecrosis at the site of the envenomation and in cases of systemic illness supportive care is recommended. Therapeutic plasma exchange has been shown efficacious in treating snake envenomation, but there are limited data detailing its use for brown recluse spider envenomation. Here we present a case to highlight the benefit of plasmapheresis in a patient with acute hemolytic anemia secondary to a brown recluse spider bite.
Keywords: Loxoscelism; Hemolysis; Plasma exchange; Brown recluse spider
| Introduction | ▴Top |
Loxosceles reclusa (L. reclusa), better known as the brown recluse spider, is a small hunting spider commonly found in the central and southeastern parts of the USA [1]. Loxoscelism denotes systemic effects from the L. reclusa venom. Most of the clinical manifestations are limited to dermonecrotic effects [2]. Although less common, brown recluse bites, can result in significant morbidity ranging from hemolytic anemia, disseminated intravascular coagulation and acute renal failure, occasionally leading to multiorgan failure and death [3]. The recluse spider venom contains various enzymes enriched in phospholipase D, sphingomyelinase, astacin-like metalloproteases, and inhibitor cystine knot peptides [4]. Phospholipase D is unique to Loxosceles, and it works directly or indirectly to cytotoxic activities upon different cells by activating complement, inducing neutrophil chemotaxis, apoptosis of keratinocytes causing dermatonecrosis, hemolytic activity on erythrocytes, platelet aggregation and renal failure [4, 5]. The brown recluse spider is usually found in dark, warm, dry basements in both rural and urban homes in endemic areas [6]. The spider that causes the envenomation is often not identified and there are no available commercial tests to detect spider venom [4, 6]. Based upon the history and physical exam findings, a presumptive diagnosis of a spider bite is inferred. The diagnosis however is often missed or mislabeled as cellulitis or other skin infection [4]. The management of brown recluse spider bites is primarily supportive as there is no definitive intervention or guideline treatment [1, 4]. Supportive care usually entails red blood cell transfusions, intravenous fluids, and local wound care [2]. In more severe cases, other treatments have included hyperbaric oxygen, dapsone, antivenom, steroids and intravenous immunoglobulin (IVIG) with variable success [2, 4]. Therapeutic plasma exchange or plasmapheresis is an extracorporeal method to remove substances from the blood. Plasmapheresis works to separate plasma from cellular blood components with replacement of crystalloid or plasma from a donor. Plasmapheresis has been used to treat snake envenomation [1]. We present this case to highlight the benefit of plasmapheresis in this case of acute hemolytic anemia caused by a brown recluse spider bite.
| Case Report | ▴Top |
Investigations
A previously healthy 49-year-old male presented to the emergency department due to a worsening spider bite on his right shoulder (Fig. 1). He reported getting the bite 5 days prior while putting up sheet rock at work. He had not seen the spider but reported “backing up into a spider web.” He was seen twice before in the emergency department in the past week.
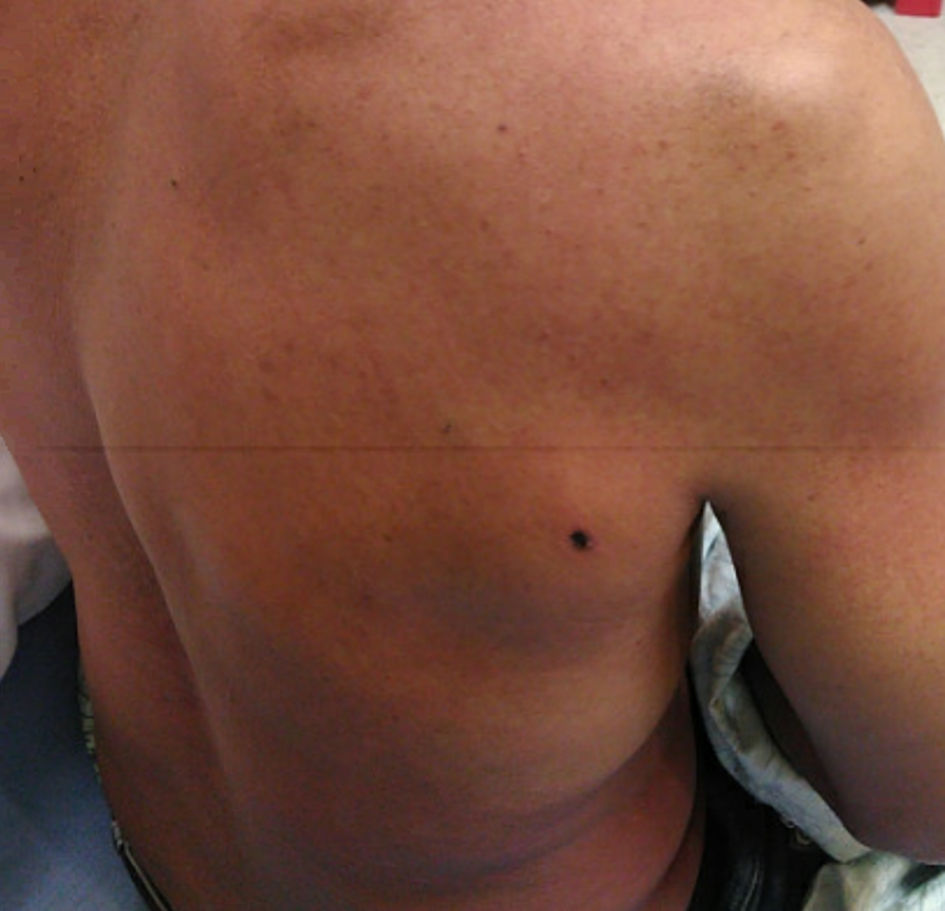 Click for large image | Figure 1. Skin lesion at initial presentation to emergency room: 1-cm black scab with surrounding induration without fluctuance. |
At his initial presentation to the emergency room, his laboratory evaluation showed a normal renal function; however, he did have mild elevation in the aspartate aminotransferase (AST) at 64 U/L, alanine aminotransferase (ALT) 56 U/L, total bilirubin 1.1 mg/dL, white blood cell count 12.3 × 103/mm3, hemoglobin14.7 g/dL, platelet count 129 × 103/mm3. His urine was noted to be orange in appearance with no blood on the analysis. He was given a prescription for doxycycline but did not get it filled.
He represented to the emergency room 3 days later for worsening of his rash, fever, body aches and discoloration of his urine. He was noted be febrile at 40.5 °C, heart rate of 103 beats/min, blood pressure 137/96 mm Hg, respirations at 19 breaths/min, oxygen saturation of 98% on room air. Laboratory evaluation was significant for normal creatinine at 1.09 mg/dL, total bilirubin of 5.7 mg/dL with noted hemolysis on fractionation, AST 50 U/L, ALT 82 U/L, creatinine kinase 265 U/L, white blood cell count 16.3 × 103/mm3, hemoglobin 11.3 g/dL, platelet count 112 × 103/mm3, and lactic acid 1.4 mmol/L. The patient was noted to meet sepsis criteria and it was recommended he be admitted to the hospital for intravenous antibiotics; however, the patient refused.
He returned again to the emergency room 2 days later with pain around the bite with extension to his chest, fevers, body aches, shortness of breath, nausea, and vomiting (Fig. 2). Initial vitals were a temperature of 36.9 °C, heart rate 94 beats/min, respiratory rate 18 breaths/min, blood pressure 127/80 mm Hg, and oxygen saturation of 97% on room air. The patient’s urine was noted to be black colored at bedside.
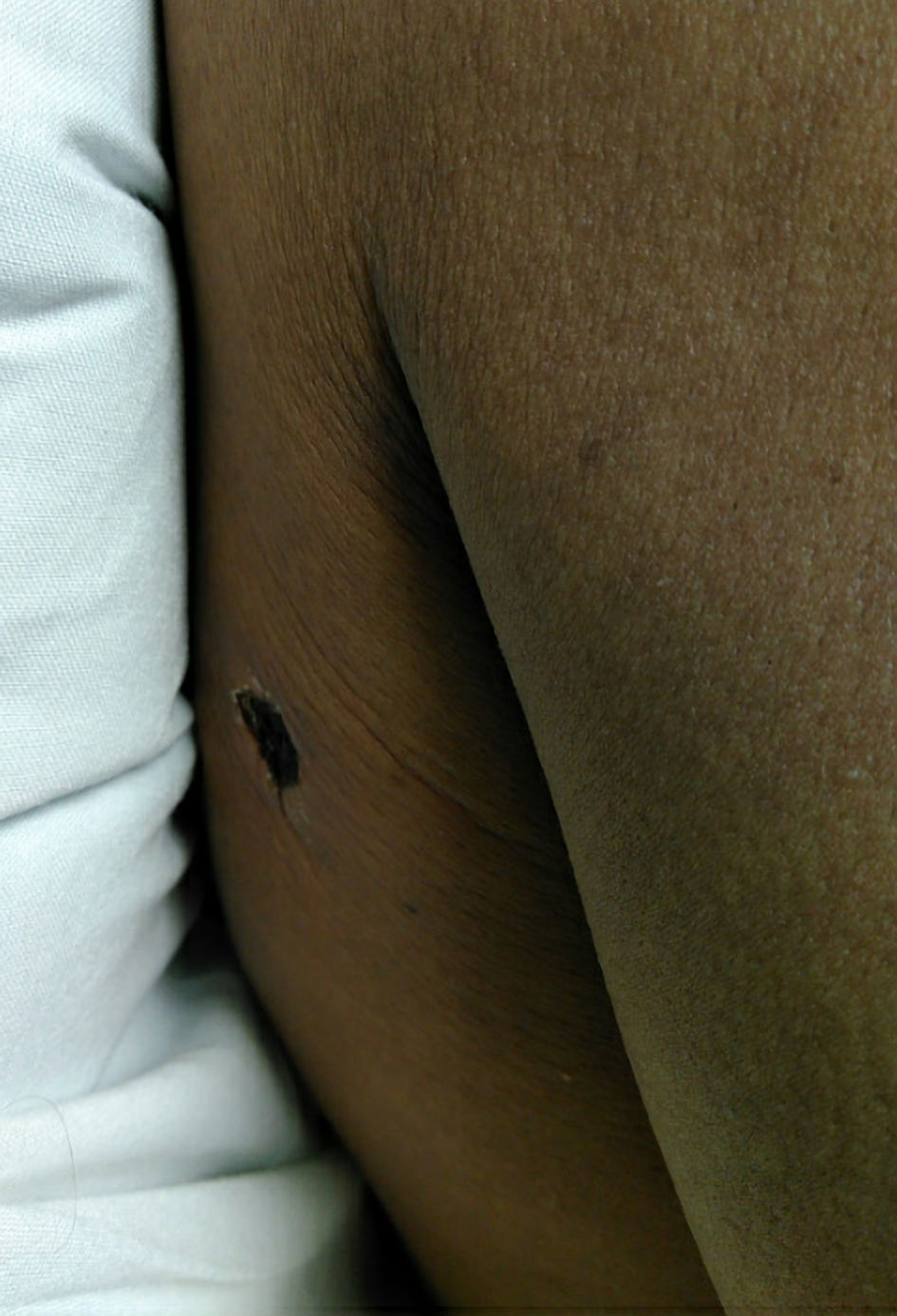 Click for large image | Figure 2. Skin lesion at hospital admission. Black eschar to right posterior shoulder about 1 cm in diameter with mild surrounding erythema without induration or fluctuance. |
Diagnosis
Laboratory evaluation showed significant hemolysis. His bicarbonate level was 18 mmol/L, blood urea nitrogen (BUN) 107 mg/dL, creatinine 6.46 mg/dL, total bilirubin 5.1 mg/dL with noted hemolysis on fractionation, AST 74 U/L, ALT 50 U/L, creatinine kinase 624 U/L, lactate dehydrogenase 2,171 U/L, haptoglobin < 10 mg/dL, white blood cell count 32 × 103/mm3, hemoglobin 5.9 g/dL, platelet count 171 × 103/mm3, reticulocyte count 4.3%, and lactic acid 1.6 mmol/L. Urine was noted to be brown/black with large amount of blood present. Direct Coombs test was immunoglobulin G (IgG) negative and C3 positive. A peripheral smear showed normocytic anemia with spherocytes and many nucleated red blood cells, leukocytosis with left shifted neutrophil series, and eosinophilia.
He was given intravenous vancomycin and piperacillin/tazobactam in the emergency room and admitted to the intensive care unit for further evaluation and treatment. Nephrology was consulted for acute renal failure likely secondary to high load of pigment injury. An accurate potassium level could not be obtained with the hemolysis. The patient was nonoliguric and no other indications for hemodialysis was present, nephrology recommended continued hemodynamic support. Antibiotics were de-escalated to ceftriaxone 1 g every 24 h and continued on intravenous vancomycin. General surgery was consulted for evaluation of spider bite and their recommendation was supportive care with no need for any debridement. Poison control was contacted; however, because the bite occurred 1 week prior he was outside the time window for receiving any antivenom. He was transfused two units packed red bloods cell upon admission with hemoglobin/hematocrit evaluation every 6 h with goal to transfuse if hemoglobin less than 7 g/dL.
Hematology was consulted for the acute hemolytic anemia. Peripheral smear was reviewed showing no evidence of thrombotic microangiopathy with no schistocytes identified. The Coombs test was positive for acute hemolytic anemia. Recommendation was for supportive care with transfusions for hemoglobin less than 7 g/dL and holding on initiating plasmapheresis. There was discussion for using steroids, but since the hemolysis was complement mediated there would be no role in its utility.
Over the next 2 days the patient continued to decline with worsening laboratory evaluation including bicarbonate level of 15 mmol/L, BUN 144 mg/dL, creatinine 9.35 mg/dL, total bilirubin 2.6 mg/dL with noted hemolysis on fractionation, AST 112, ALT 47, hemolyzed creatinine kinase and lactic acid dehydrogenase, white blood cell 38 × 103/mm3, hemoglobin 5.8 g/dL, and platelet count 148 × 103/mm3. Cold agglutinins were not detected.
Treatment
He was transfused an additional two units of packed red blood cells. Nephrology noted worsening acute kidney injury with uremic symptoms including nausea, vomiting, oliguria, and recommended placement of a hemodialysis line and to begin intermittent hemodialysis.
On hospital day 2, given the worsening acute renal injury, continued hemolysis, and transfusion dependence, we recommended initiation of plasmapheresis with a one-to-one exchange with albumin (Fig. 3). This was discussed with the blood bank. The patient was transfused one unit packed red blood cells overnight after plasmapheresis. The following day after plasmapheresis his laboratory evaluation revealed a bicarbonate level of 18 mmol/L, BUN 93 mg/dL, creatinine 7.69 mg/dL, total bilirubin of 1.3 mg/dL with indirect bilirubin of 0.8 mg/dL, AST 51 U/L, ALT 20 U/L, creatinine kinase 227 U/L, white blood cell count 34.6 × 103/mm3, hemoglobin 7.0 g/dL, platelet count 146 × 103/mm3. The plan was to continue with plasmapheresis every other day. Given the worsening leukocytosis, antibiotics were broadened to clindamycin and cefepime and continued intravenous vancomycin. A repeat ultrasound at the site of the spider bite was done with no evidence of a fluid collection or abscess.
 Click for large image | Figure 3. Patient’s plasma seen during the first plasmapheresis session. |
The patient underwent a second treatment of plasmapheresis again with a one-to-one exchange with albumin (Fig. 4). Laboratory examination the following day revealed improvement with creatinine 7.06 mg/dL, white blood cell count 20.9 × 103/mm3, hemoglobin 8.2 g/dL, and platelet count 147 × 103/mm3. No additional blood transfusions were needed, and given the clinical and laboratory improvement, no additional plasmapheresis was recommended. It was recommended the patient start folic acid 2 mg daily for 30 days. He underwent three sessions total of hemodialysis and was stopped after creatinine continued to improve and had adequate urine output. Supportive management was recommended for his bite wound.
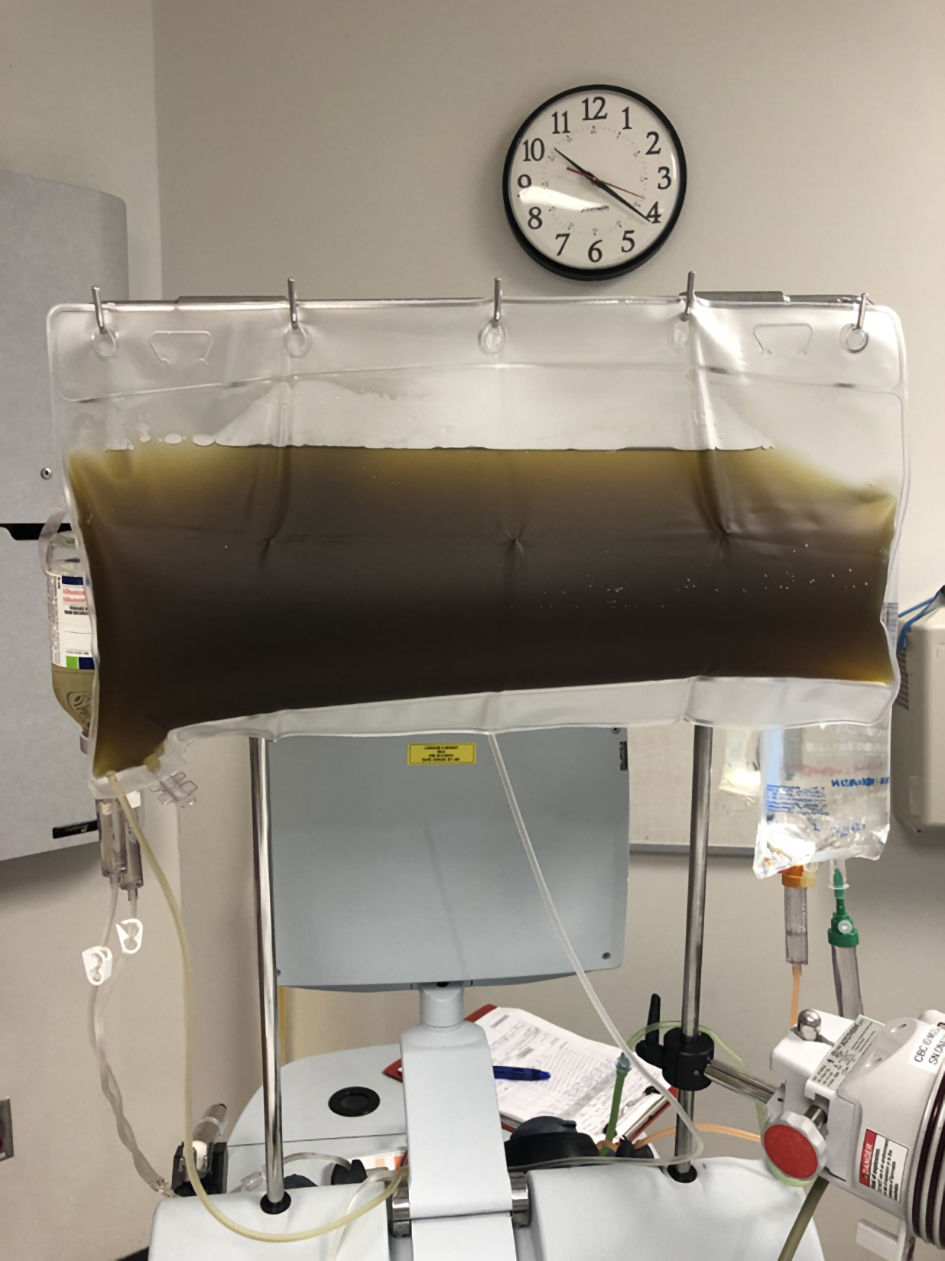 Click for large image | Figure 4. Patient’s plasma with the second plasmapheresis session. |
Follow-up and outcomes
Due to his improvement in clinical status, he was discharged with instructions for close follow-up and outpatient labs. On the day of discharge, his creatinine was 5.59 mg/dL, white blood cell count 8.8 × 103/mm3, hemoglobin 8.1 g/dL, and platelet count 320 × 103/mm3 (Fig. 5).
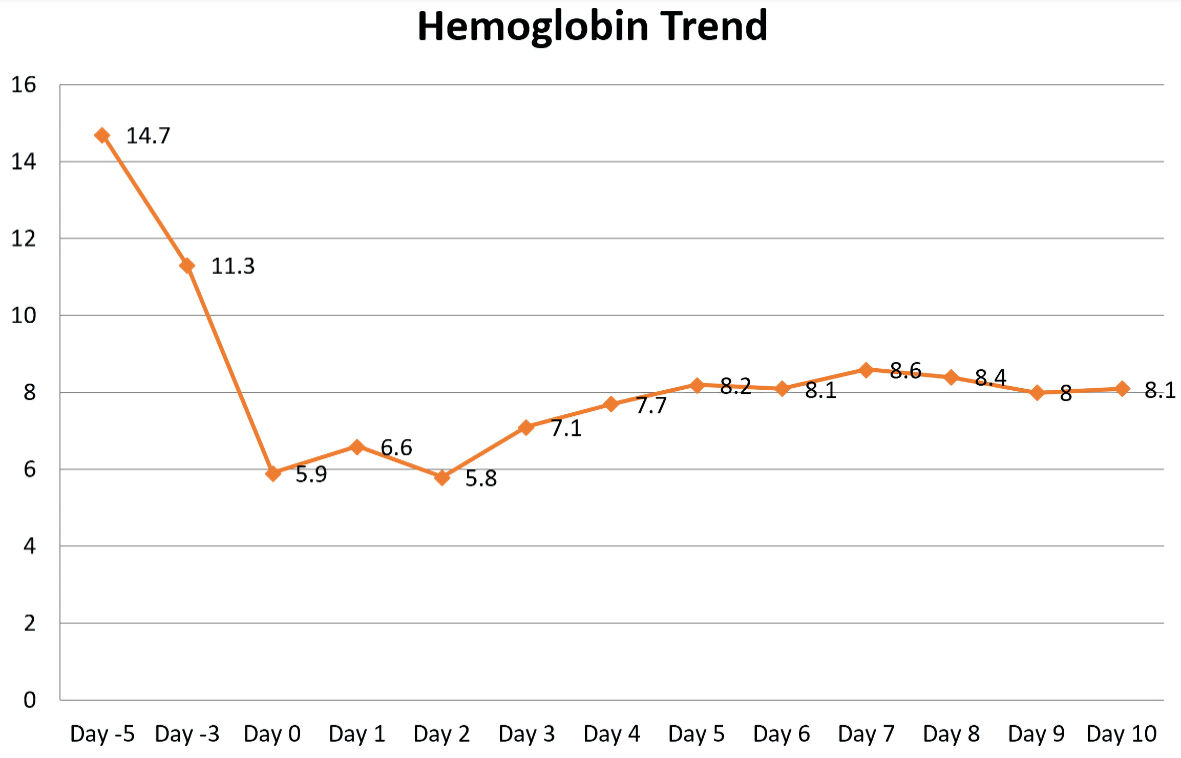 Click for large image | Figure 5. Plasmapheresis started on hospital day 2. |
| Discussion | ▴Top |
Our patient represents an uncommon complication following a brown recluse spider bite. The patient had presented to the emergency room with worsening pain around the site of a likely spider bite. His laboratory exam at that time was generally unremarkable and he was treated for cellulitis with antibiotics. However, several days after his initial presentation, he re-presented to the emergency room with worsening pain at the site of the spider bite and more symptoms concerning for multisystemic disease including hemolytic anemia with acute kidney failure. Given the patient’s refractory hemolytic anemia, plasmapheresis was attempted with success. This report gives an overview of the published evidence available on brown recluse spider bite and the utility of plasmapheresis.
The brown recluse spider, L. reclusa is the most common representative of the nine species of brown spiders found in the USA [7]. It is responsible for most of the episodes of envenomation in its endemic region in the southern half of the Missouri-Ohio-Mississippi River basin [6, 7]. They commonly inhabit outside and inside isolated environments including closets, attics, and basements. Most of the bites occur when they feel endangered [4]. Our patient described this common scenario for his own bite as he was working in the basement and had backed up into a spider web likely causing the spider to feel threatened, consequently, leading to the bite. Generally, there is little pain associated with the spider bite until about 2 - 6 h after envenomation, thus the spider is rarely seen by the victim and the diagnosis is usually presumptive and made through epidemiological information and evolution of the clinical picture [7, 8]. The clinical picture associated with L. reclusa bite is characterized by an intense inflammatory reaction and dermatonecrosis at the bite site [9]. This was the presentation of the patient with the first emergency room visit with local irritation surrounding the site of the bite. Loxoscelism is the term used for the severe cases where systemic manifestations develop including intravascular hemolysis, disseminated intravascular coagulation and acute renal failure [6]. It was 5 days after the spider bite when our patient returned to the emergency room with signs and symptoms consistent with systemic loxoscelism including acute hemolysis and renal failure.
The venom of a L. reclusa spider is a mixture of proteic toxins enriched with molecules of low molecular mass [5]. The toxins include alkaline phosphatase, ribonucleotide phosphohydrolase, hyaluronidase, serine proteases, metalloproteases and sphingomyelinase-D. These toxins play a role in causing hemostatic disturbances including injury to the blood vessels, hemorrhage into the dermis, imperfect platelet adhesion, defective wound healing, gravitational spreading of dermatonecrosis as well as systemic spread [5]. The most characterized component of the venom is sphingomyelinase D which is thought to play a key role is the clinical manifestation of loxoscelism including dermatonecrosis, platelet aggregation and complement-dependent hemolytic activities through direct toxin-induced hemolysis and complement-mediated immune destruction [4, 6]. In our patient, evidence of acute hemolysis was present upon admission to the hospital. The patient had markers of hemolysis with elevated lactate dehydrogenase, indirect hyperbilirubinemia, low haptoglobin levels, and reticulocytosis. Peripheral smear revealed evidence of increased red cell turnover with abundance of immature red blood cells. His direct antiglobulin test was positive for C3 and negative for IgG suggesting complement-mediated immune destruction. Additionally, the venom is a potent endothelial agonist inducing E-selectin expression, stimulating the release of interleukin (IL)-8 and large amounts of granulocyte-macrophage colony-stimulating factor (GM-CSF), and inducing neutrophil chemotaxis [6]. This could in part explain the leukemoid reaction that was seen in our patient with a white blood cell count peaking around 40,000.
Typically, envenomation from L. reclusa results in a cytotoxic reaction that manifests as a localized cutaneous response. The bite initially presents with transient erythema followed by development of a blister with surrounding induration and erythema. Usually, within 24 h the area of the bite turns violaceous to black in color and by the seventh day the central area becomes dark and necrotic [7]. Rarely, less than 10% of patients can develop systemic reactions in addition to local symptoms [10]. Systemic loxoscelism can develop 7 - 10 days after the bite resulting in symptoms such as fever, rash, jaundice, nausea, vomiting, headaches, abdominal pain and in severe cases hemolysis, coagulopathy, nephrotoxicity, disseminated intravascular coagulopathy and even death [1, 4]. Our patient presented 5 days after obtaining the spider bite with similar symptoms including fevers, body aches, shortness of breath, nausea and vomiting along with acute renal failure and anemia secondary to severe hemolysis.
Treatment following the bite of a L. reclusa spider is generally supportive care. For the localized cutaneous response, wound care should be sought with consideration of surgical debridement for skin necrosis [4]. However, the appropriate treatment for systemic loxoscelism is unclear and poorly studied. Red blood cell transfusions are indicated for symptomatic anemia related to hemolysis, with most patients requiring around three units packed red blood cells [4]. Different therapies have been tried including high-dose steroids, systemic antimicrobials, antihistamines, vasodilators, urinary alkalization, exchange transfusion and plasmapheresis [1, 4]. High-dose steroids have been tried in moderate to severe hemolysis in an attempt to treat complement-mediated hemolysis; however, evidence is sparse regarding its efficacy in the management of this complication [1, 4]. In this case report, our patient required four units of packed red blood cells prior to initiation of plasmapheresis.
Plasmapheresis is used to remove substances from the blood such as antibodies, immune complexes, complement components and various cytokines. It is extracorporeal and functions by removing the patient’s own plasma from other blood components and replacing it with albumin or fresh frozen plasma [1]. In the hematology realm, plasmapheresis is the treatment of choice for thrombotic thrombocytopenic purpura by removing antibodies that block ADAMTS13 and replace these enzymes. Plasmapheresis has reported use in snake envenomation. Yildirim et al described using plasmapheresis to treat 16 cases of snake envenomation in patients who did not receive antivenom. In their documented experience, plasmapheresis was used with human albumin as the main replacement fluid. The average number of plasmapheresis sessions was 1.9 per patient with average length of hospital stay of 16.6 days. They reported rapid improvements in coagulopathies after initiation of plasmapheresis and attributed salvage of limbs in victims of snake bites with early use of plasmapheresis [11]. In a case report by Arora et al, they highlighted the use of plasmapheresis in the setting of acute kidney injury with severe thrombocytopenia after a patient was bitten by a Russell’s viper snake. Plasmapheresis was initiated after persistent hemolysis with acute kidney failure requiring hemodialysis and refractory to multiple blood transfusions, high-dose steroids and anti-serum venom. Plasmapheresis was done with 5% albumin and fresh frozen plasma. Eight hours after completion of plasmapheresis, the patient’s laboratory examinations stared improving with stability of the hemoglobin and rapid improvement in the thrombocytopenia. One additional plasmapheresis session was planned 24 h later, but the patient and family refused due to financial constraints. However, the patient’s hemoglobin and platelet count had stopped falling and all markers of hemolysis were within normal limits [12]. There is very limited evidence on the use of plasmapheresis for L. reclusa envenomation. Abraham et al described using plasmapheresis for a 17-year-old female with refractory hemolysis after a brown recluse spider bite that was treated with plasma exchange. In this particular case, the patient had been treated with high-dose steroids and discharged on oral prednisone but re-presented 24 h later with worsening hemolysis. She was initiated on plasmapheresis with rapid clinical and laboratory status improvement without need for further transfusions [1]. In addition, plasma exchange has been successfully used to clear the plasma to allow the ability to perform routine laboratory assessments. In a case described by Said et al, a 6-year-old boy presented with systemic loxoscelism with massive hemolysis, disseminated intravascular coagulopathy, shock and acute renal failure. The patient’s severe hemoglobinemia prohibited routine laboratory measurements due to how opaque the plasma had become from the hemolysis. Plasmapheresis was successfully used to clear the plasma and restore the ability to perform laboratory assessments along with correcting the underlying coagulopathy [2].
Learning points
In conclusion, this case report illustrates several key points. The diagnosis of an L. reclusa bite can easily be misdiagnosed as cellulitis, and clinicians should pay close attention to the history especially in an area endemic to these spiders. The diagnosis of loxoscelism, although rare, should not be missed, and close follow-up for systemic manifestations is warranted. The standard of care for patients who develop systemic symptoms related to the spider bite is supportive care including red blood cell transfusions for hemolytic anemia. However, for the patients who develop systemic loxoscelism with refractory hemolysis, consideration should be given to a trial of plasmapheresis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Verbal informed consent was obtained.
Author Contributions
Stephanie Harry helped draft the entire case report, edit the manuscript, and make the Figure 5. Emily Brugioni helped draft the entire case report, edit the manuscript, and make Figures 1 - 4. Sheshadri Madhusudhana contributed to the editing of case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Abraham M, Tilzer L, Hoehn KS, Thornton SL. Therapeutic plasma exchange for refractory hemolysis after brown recluse spider (Loxosceles reclusa) envenomation. J Med Toxicol. 2015;11(3):364-367.
doi pubmed - Said A, Hmiel P, Goldsmith M, Dietzen D, Hartman ME. Successful use of plasma exchange for profound hemolysis in a child with loxoscelism. Pediatrics. 2014;134:1464-1467.
doi pubmed - McDade J, Aygun B, Ware RE. Brown recluse spider (Loxosceles reclusa) envenomation leading to acute hemolytic anemia in six adolescents. J Pediatr. 2010;156(1):155-157.
doi pubmed - Nguyen N, Pandey M. Loxoscelism: cutaneous and hematologic manifestations. Adv Hematol. 2019;2019:4091278.
doi pubmed - Chaim OM, Sade YB, da Silveira RB, Toma L, Kalapothakis E, Chavez-Olortegui C, Mangili OC, et al. Brown spider dermonecrotic toxin directly induces nephrotoxicity. Toxicol Appl Pharmacol. 2006;211(1):64-77.
doi pubmed - Anwar S, Torosyan R, Ginsberg C, Liapis H, Morrison AR. Clinicopathological course of acute kidney injury following brown recluse (Loxoscles reclusa) envenomation. Clin Kidney J. 2013;6(6):609-612.
doi pubmed - Foil LD, Norment BR. Envenomation by Loxosceles reclusa. J Med Entomol. 1979;16(1):18-25.
doi pubmed - Malaque CM, Santoro ML, Cardoso JL, Conde MR, Novaes CT, Risk JY, Franca FO, et al. Clinical picture and laboratorial evaluation in human loxoscelism. Toxicon. 2011;58(8):664-671.
doi pubmed - Magalhaes GS, Caporrino MC, Della-Casa MS, Kimura LF, Prezotto-Neto JP, Fukuda DA, Portes-Junior JA, et al. Cloning, expression and characterization of a phospholipase D from Loxosceles gaucho venom gland. Biochimie. 2013;95(9):1773-1783.
doi pubmed - Ginsburg CM, Weinberg AG. Hemolytic anemia and multiorgan failure associated with localized cutaneous lesion. J Pediatr. 1988;112(3):496-499.
doi - Yildirim C, Bayraktaroglu Z, Gunay N, Bozkurt S, Kose A, Yilmaz M. The use of therapeutic plasmapheresis in the treatment of poisoned and snake bite victims: an academic emergency department's experiences. J Clin Apher. 2006;21(4):219-223.
doi pubmed - Arora P, Belwal S, Uniyal B, Saxena S. Plasmapheresis in a case of acute kidney injury with severe hemolysis and thrombocytopenia due to hematotoxic (Russell's viper) snake bite. Saudi J Kidney Dis Transpl. 2020;31(1):276-280.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


