| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 1, January 2022, pages 21-25
A Case of Type 2 Diabetes Mellitus With t(6;7)(q24;q31.2) Balanced Translocation
Chiharu Choa, Kazuki Kodoa, b , Sachiko Gotoa, Yoshiki Katsumia
aDepartment of Pediatrics, Saiseikai Kyoto Hospital, Nagaokakyo, Kyoto, Japan
bCorresponding Author: Kazuki Kodo, Department of Pediatrics, Saiseikai Kyoto Hospital, 8 Minami-hirao, Imazato, Nagaokakyo city, Kyoto 617-0814, Japan
Manuscript submitted November 12, 2021, accepted December 14, 2021, published online January 17, 2022
Short title: T2DM With t(6;7)(q24;q31.2)
doi: https://doi.org/10.14740/jmc3843
| Abstract | ▴Top |
Most balanced translocations do not involve any gain or loss of genetic material, and individuals harboring these translocations remain clinically asymptomatic. Nevertheless, balanced translocations have reportedly been associated with several diseases. Here, we present the case of a 12-year-old boy with type 2 diabetes mellitus that could not be explained only by obesity; the patient harbored a balanced translocation (46,XY t(6;7)(q24;q31.2)). Interestingly, genetic analysis showed that his 10-year-old sister also carried the same translocation and shared the same symptoms. Further analyses are required to confirm whether this balanced translocation is associated with the symptoms presented in our patient and his sibling. The outcomes of our case study are expected to reveal novel loci causing diabetes and have implications for improved diagnosis and treatment.
Keywords: Balanced translocation; Type 2 diabetes mellitus; Metabolic syndrome; G-banding
| Introduction | ▴Top |
Chromosomal translocation is one of the most common types of chromosomal abnormalities. There are two types of chromosomal translocation: balanced and unbalanced translocations [1]. It is difficult to detect balanced translocations because most balanced translocations do not involve the gain or loss of genetic information, and individuals carrying these genes do not exhibit clinical symptoms [2]. However, several studies have revealed associations between short stature, developmental delay, and facial malformation with balanced translocations [3-5]. Further, a rare case of a patient with abnormal phenotypes, such as diabetes mellitus (DM), has been reported [6]. The characterization of disease-associated balanced translocations has led to the detection of genes responsible for various disorders, including various forms of DM. Here, we present the case of a 12-year-old boy with type 2 DM (T2DM) carrying a balanced translocation (46,XY t(6;7)(q24;q31.2)).
| Case Report | ▴Top |
Investigations
The patient was a 12-year-old Japanese boy. He was referred to our hospital owing to the detection of glycosuria during urinary screening at his school. He was born at 41 weeks of gestational age. His birth weight and height were 3,028 g (-0.6 standard deviation (SD)) and 51 cm (+0.6 SD), respectively. He had no medical history of hyperglycemia during infancy. However, when he began elementary school, he had a medical history of autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) for which he has received no medication to date. His score on the matrix reasoning subtest of the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-4) was 90 (average range: 85 - 115). His parents had normal physique and did not have DM. His 10-year-old sister had ASD, ADHD, and was obese.
Diagnosis
On physical examination, the body weight of the patient was 79.8 kg (+2.6 SD) and his height was 163.1 cm (+1.8 SD). His body mass index (BMI) was 29.9 kg/m2 (+2.19 SD, 98.6th percentile), and his waist circumference was 94.5 cm. Systolic blood pressure (SBP) was 118 mm Hg and diastolic blood pressure (DBP) was 70 mm Hg (criteria for metabolic syndrome in Japanese children are SBP ≥ 125 mm Hg and/or DBP ≥ 70 mm Hg [7]). Laboratory investigations revealed a fasting plasma glucose level of 131 mg/dL and glycated hemoglobin (HbA1c) value of 7.3%. The homeostasis model assessment of insulin resistance (HOMA-IR) value was 6.1 (reference range in non-obese pubertal children, < 2.6 [8]). Autoantibodies against glutamic acid decarboxylase and islet antigen type 2 were negative. He did not have any endocrine abnormalities leading to insulin resistance, and his serum growth hormone, cortisol, free triiodothyronine, and free thyroxin levels were 0.13 ng/mL, 20.9 µg/dL, 3.2 pg/mL, and 1.14 ng/dL, respectively. His serum triglyceride, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels were 159, 111, and 48 mg/dL, respectively. His liver enzyme levels were elevated; aspartate aminotransferase and alanine aminotransferase levels were 58 and 106 IU/L, respectively. Computed tomography revealed fatty liver and visceral fat accumulation. Therefore, he was diagnosed with T2DM and metabolic syndrome. Genetic examination was requested because he had a mild developmental disorder. After we obtained informed consent from the patient and his parents, we conducted a genetic analysis for excluding chromosomal abnormalities (e.g., Prader-Willi syndrome). G-banding and fluorescence in situ hybridization (FISH) were performed, and we identified a 46,XY t(6;7)(q24;q31.2) chromosomal translocation (Fig. 1a). To determine the origin of the reciprocal translocation, genetic examinations of the family members of the proband were performed. The proband’s father and sister had the same chromosomal translocation, while his mother had a normal karyotype (Fig. 1b). His father was phenotypically normal, but his sister was obese and had a mild developmental disorder, similar to the proband. Chromosome 6q24, the paternal allele of the imprinted gene locus, is closely related to diabetes [9, 10], while chromosome 7q31-33 is strongly linked to autism [11]; therefore, we additionally performed array comparative genomic hybridization (CGH). However, the results of array CGH did not show any copy number variation in the breakpoint regions on chromosomes 6 and 7 (Fig. 2a). The methylation coefficient of pleiomorphic adenoma gene-like 1 (PLAGL1) located on chromosome 6q24 was analyzed. However, no abnormal methylation was detected (Fig. 2b).
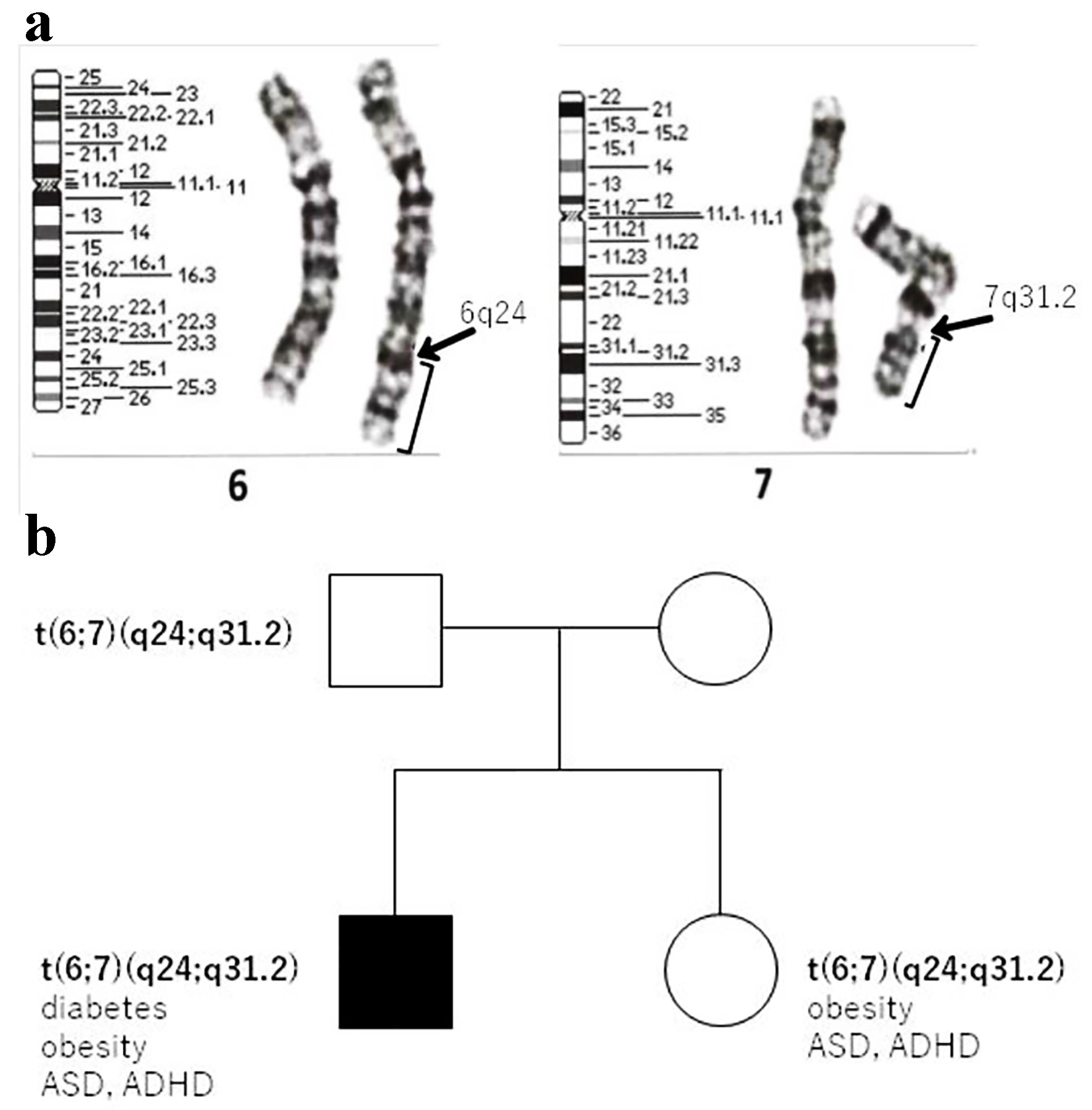 Click for large image | Figure 1. (a) Cytogenetic analysis showing a balanced reciprocal translocation between the long arms of chromosomes 6 and 7. (b) Family pedigree of the patient. T2DM: type 2 diabetes mellitus; ASD: autism spectrum disorder; ADHD: attention deficit hyperactivity disorder. |
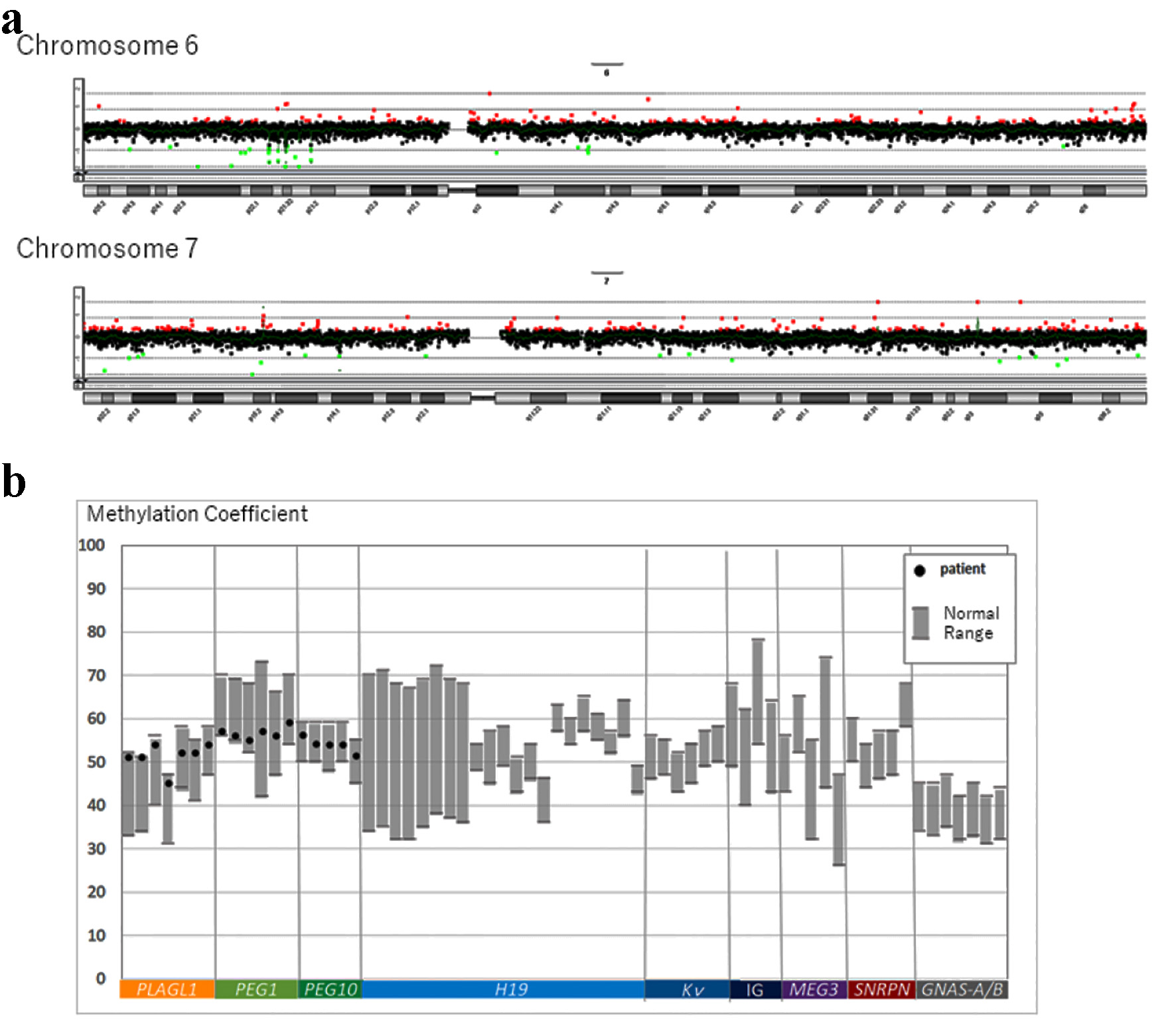 Click for large image | Figure 2. (a) Summary of copy number alterations in chromosomes 6 and 7 detected by array CGH. (b) Screening for genomic imprinting disorders for the methylation coefficient of PLAGL1, PEG1, and PEG10 using the pyrosequencing method. PLAGL1: related to neonatal DM. PEG1 and PEG10: related to Silver-Russell syndrome. CGH: comparative genomic hybridization. |
Treatment
We initiated planned diet and exercise therapy to mitigate the symptoms of T2DM and the metabolic syndrome. After lifestyle modification, the glycated hemoglobin (HbA1c) value of the patient temporarily decreased to 6.5%. However, HbA1c value increased from 6.5% to 7.2% over a 2-month interval. Subsequently, we initiated treatment with metformin (administered as two 250 mg doses per day).
Follow-up and outcomes
After 6 months of treatment with metformin, the HbA1c value of the proband improved to 6.1%, and his body weight reduced to 74.8 kg.
| Discussion | ▴Top |
It is assumed that gene breakages, which lead to the formation of balanced translocations, do not cause a marked loss of chromosomal segments; consequently, considerable gene loss and phenotypic changes in the individuals do not occur [2]. Nevertheless, balanced translocations lead to various chromosomal anomalies that can be detected in patients with abnormal phenotypes as well as healthy individuals who are asymptomatic [1]. Previous studies have reported the association of short stature and developmental delay with balanced translocations [3, 4]. Furthermore, a previous case study has suggested an association between balanced translocation and maturity-onset diabetes of the young (MODY) [6].
To the best of our knowledge, the present study is the first to report the association of balanced reciprocal translocation in chromosomes 6 and 7 with T2DM in a patient. We observed that the same translocation was harbored by the patient’s father and sister, and his sister had a similar phenotype with elevated HOMA-IR values. These considerations render this case even more interesting from a clinical point of view, as they indicate a correlation between supposedly harmless translocations and effect in predisposing carriers to certain health conditions.
There are several possible causes of symptomatic balanced translocations. Balanced translocations can become imbalanced via de novo regulation at the molecular level, uniparental disomy, disruption of genes located at the breakpoint, and undetected mosaicism in the tissue [3]. These minimal changes cannot be analyzed using G-banding and FISH. Both PLAGL1 and hydatidiform mole associated and imprinted (HYMAI) located on chromosome 6q24 are closely related to diabetes as the paternal allele of the imprinted locus [9, 10]. The whole-genome data from multiple families implicate the interactions among at least 10 genes on chromosome 7q31 in the causation of autism [11]. We performed array CGH; we did not observe any copy number variation in the two imprinted genes on chromosomes 6 and 7.
Further investigations are required to determine the whole genome sequence and sample target tissues to identify any associated mosaicism. In this case, unfortunately, we could not exclude another gene anomaly. We could not perform whole genome sequencing in our patient, because the family refused this request, owing to socio-economic reasons.
In conclusion, we encountered a case of T2DM with t(6;7)(q24;q31.2) balanced translocation. Importantly, in the case of a reciprocal translocation with T2DM as a phenotypic manifestation, further analyses are warranted to obtain additional information that will aid in making an accurate diagnosis and providing effective treatment.
Learning points
In clinical practice, we often encounter children with obesity. However, not all children with obesity develop DM. Some children develop DM even after minimal weight gain. Presumably, obesity exacerbates the development of DM, but it is possible that other factors are responsible for impaired glucose tolerance. The discovery of chromosomal abnormalities can contribute to the development of therapies for various diseases. Ultimately, when a patient is found to harbor a balanced translocation, the most important consideration is genetic counseling for the patient and their family to detect the prevalence of any familial genetic conditions.
Acknowledgments
We would like to thank Dr. Maki Fukami from the National Center for Child Health and Development for helping us with array comparative genomic hybridization.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient as well as his parents for participation in this study and the use of patient data for publication purposes. The patient information was de-identified for publication.
Author Contributions
CC and KK mainly examined the patient and drafted the initial manuscript. GS and KY helped with the clinical treatment, reviewed, and revised the manuscript. All authors have read and approved the final manuscript.
Data Availability
The data supporting the findings of this case study are openly available from the authors.
Abbreviations
DM: diabetes mellitus; T2DM: type 2 diabetes mellitus; ASD: autism spectrum disorder; ADHD: attention deficit hyperactivity disorder; WISC-4: Wechsler Intelligence Scale for Children-Fourth Edition; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HbA1c: glycated hemoglobin; HOMA-IR: homeostasis model assessment of insulin resistance; FISH: fluorescence in situ hybridization; CGH: comparative genomic hybridization; MODY: maturity-onset diabetes of the young; PLAGL1: pleiomorphic adenoma gene-like 1; HYMAI: hydatidiform mole associated and imprinted
| References | ▴Top |
- Jacobs PA, Browne C, Gregson N, Joyce C, White H. Estimates of the frequency of chromosome abnormalities detectable in unselected newborns using moderate levels of banding. J Med Genet. 1992;29(2):103-108.
doi pubmed - Warburton D. De novo balanced chromosome rearrangements and extra marker chromosomes identified at prenatal diagnosis: clinical significance and distribution of breakpoints. Am J Hum Genet. 1991;49(5):995-1013.
- Akbas A, Orhan K, Capan K, Mahmut B, Turgay B. Familial balanced reciprocal translocation [t(16;22) (p11;q13) mat] in a child with constitutional short stature. J Med Cases. 2012;3(2):149-152.
doi - Tonk VS, Wyandt HE, Huang X, Patel N, Morgan DL, Kukolich M, Lockhart LH, et al. Disease associated balanced chromosome rearrangements (DBCR): report of two new cases. Ann Genet. 2003;46(1):37-43.
doi - Arimura Y, Yamada C, Okada A, Yakushiji S, Okamoto N, Iida S. A case of t(11;22) (q23.3;q11.2) balanced translocation with unilateral complete cleft lip and palate. Jpn J Oral Maxillofac Surg. 2020;66(8):406-410.
doi - Bhoj EJ, Romeo S, Baroni MG, Bartov G, Schultz RA, Zinn AR. MODY-like diabetes associated with an apparently balanced translocation: possible involvement of MPP7 gene and cell polarity in the pathogenesis of diabetes. Mol Cytogenet. 2009;2:5.
doi pubmed - Yoshinaga M, Tanaka S, Shimago A, Sameshima K, Nishi J, Nomura Y, Kawano Y, et al. Metabolic syndrome in overweight and obese Japanese children. Obes Res. 2005;13(7):1135-1140.
doi pubmed - Ballerini MG, Bergada I, Rodriguez ME, Keselman A, Bengolea VS, Pipman V, Domene HM, et al. Insulin level and insulin sensitivity indices among healthy children and adolescents. Arch Argent Pediatr. 2016;114(4):329-336.
doi - Yorifuji T, Higuchi S, Hosokawa Y, Kawakita R. Chromosome 6q24-related diabetes mellitus. Clin Pediatr Endocrinol. 2018;27(2):59-65.
doi pubmed - Uchida N, Ohnishi T, Kojima T, Takahashi T, Makita Y, Fukami M, Shibata H, et al. Relapsing 6q24-related transient neonatal diabetes mellitus with insulin resistance: A case report. Clin Pediatr Endocrinol. 2020;29(4):179-182.
doi pubmed - Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472-486.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


