| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 4, April 2022, pages 172-177
Valve-in-Valve Transcatheter Aortic Valve Implantation With Acute Left and Right Coronary Artery Occlusion: A Case Report
Matjaz Bunca, c, Luka Viteza, Gian Paolo Ussiab
aDepartment of Cardiology, Division of Internal Medicine, University Medical Centre Ljubljana, Ljubljana, Slovenia
bDeparment of Cardiology, University Campus Bio-Medico, Rome, Italy
cCorresponding Author: Matjaz Bunc, Department of Cardiology, University Medical Centre Ljubljana, 1000 Ljubljana, Slovenia
Manuscript submitted November 30, 2021, accepted February 19, 2022, published online March 25, 2022
Short title: Aortic Valve Implantation With Artery Occlusion
doi: https://doi.org/10.14740/jmc3868
| Abstract | ▴Top |
Acute coronary artery occlusion is a relatively rare procedural adverse event in valve-in-valve transcatheter aortic valve implantation. Here we present a case of a 26-mm Sapien 3 prosthetic valve implantation in a degenerated 23-mm Freedom Solo bioprosthetic surgical valve with subsequent left and right coronary occlusion. Left coronary artery occlusion was managed immediately with the use of an upfront coronary artery protection technique and drug-eluting stent placement. Right coronary artery occlusion presented with right-sided heart failure and cardiac arrest that required resuscitation and additional hemodynamic support. As the artery could not be engaged with a catheter, a combination of intravenous antithrombotic and anticoagulant therapy was used as a successful bailout step to restore adequate coronary flow.
Keywords: Valve-in-valve; Transcatheter aortic valve implantation; Coronary artery occlusion; Coronary artery embolization
| Introduction | ▴Top |
Valve-in-valve (ViV) transcatheter aortic valve implantation (TAVI) has lately emerged as a relatively effective and safe treatment for patients with degenerated surgical bioprosthetic aortic valves at high surgical risk [1]. Its adverse procedural outcomes are mostly linked to device malposition and acute coronary occlusion (ACO), the latter being reported in 2-3.5% of cases [1, 2]. Obstruction is usually caused by displacement of bulky bioprosthetic valve tissue during TAVI covering the coronary ostia and is linked to significant 30-day morbidity and mortality ranging up to 53% in ViV patients [3]. Risk factors include female sex, coronary height of less than 12 mm from the annular plane and sinus of Valsalva diameter of less than 30 mm [4]. In case of ViV implantation, ACO is more common in stentless surgical bioprosthesis, stented bioprothesis with externally mounted leaflets and in patients with a virtual transcatheter valve to coronary ostia distance of less than 4 mm [3]. According to the literature, Freedom Solo stentless valve appears to have the highest rate (50%) of ACO, mainly attributed to its very elongated leaflets [5].
To prevent such complications, an upfront coronary artery protection called “chimney technique” can be used [6]. It consists of positioning a coronary guidewire, balloon or undeployed stent in the endangered artery prior to transcatheter aortic valve deployment. If coronary artery flow is compromised, a stent can then be positioned from the proximal/ostial portion of the artery up in the ascending aorta creating a channel parallel to the prosthetic valve for sufficient coronary perfusion [7].
We present a case of a ViV TAVI implantation of a Sapien 3 valve into a degenerated Freedom Solo bioprosthetic valve with subsequent left and right coronary artery occlusion.
| Case Report | ▴Top |
Investigations and diagnosis
An 89-year-old patient with a history of aortic valve replacement with a Freedom Solo 23 mm bioprosthesis (Sorin Group, Saluggia, Italy) 13 years prior was admitted to the traumatology department due to cardiogenic syncope and subsequent T1 and T2 fracture. Conservative treatment with a neck splint was advised for the thoracic vertebral fracture. As part of diagnostic workup during hospitalization, a transthoracic echocardiography (TTE) was performed that showed normal left ventricular ejection fraction, severe bioprosthetic aortic valve degeneration (valve area of 0.7 cm2, maximal velocity of 4.3 m/s, mean gradient of 42 mm Hg and Doppler velocity index of 0.22) (Fig. 1) and normal pulmonary arterial pressures. Significant coronary artery disease was excluded by coronary angiogram. He was evaluated by the local heart team and TAVI was recommended due to his high age and operative risk (STS score of 3.7%). Contrast computed tomography (CT) showed thickened and calcified bioprosthetic aortic valve leaflets with an average annulus diameter of 26 mm, annulus area of 529 mm2, a small sinus of Valsalva with a minimal diameter of 25.1 mm and relatively low coronary ostia heights (12.2 mm for the left main coronary artery and 13.1 mm for the right coronary artery) (Fig. 2). Due to the risk of coronary artery occlusion, a balloon expandable 26-mm Sapien 3 valve (Edwards Lifesciences, Irvine, CA, USA) was chosen for implantation via left transfemoral approach.
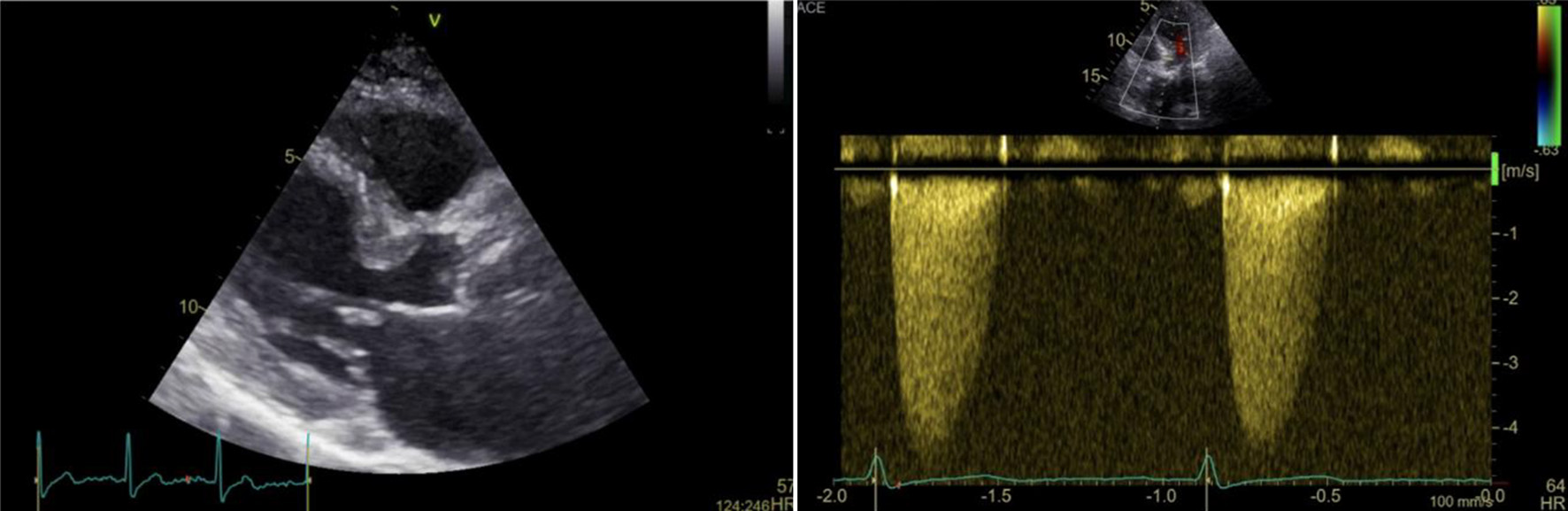 Click for large image | Figure 1. Transthoracic echocardiography of the bioprosthetic aortic valve: 2D picture showing thickened left ventricular septum and severely calcified aortic cusps (left); continuous doppler through the bioprosthetic aortic valve showing severe stenosis (maximal velocity 4.3 m/s, mean gradient 43 mm Hg) (right). |
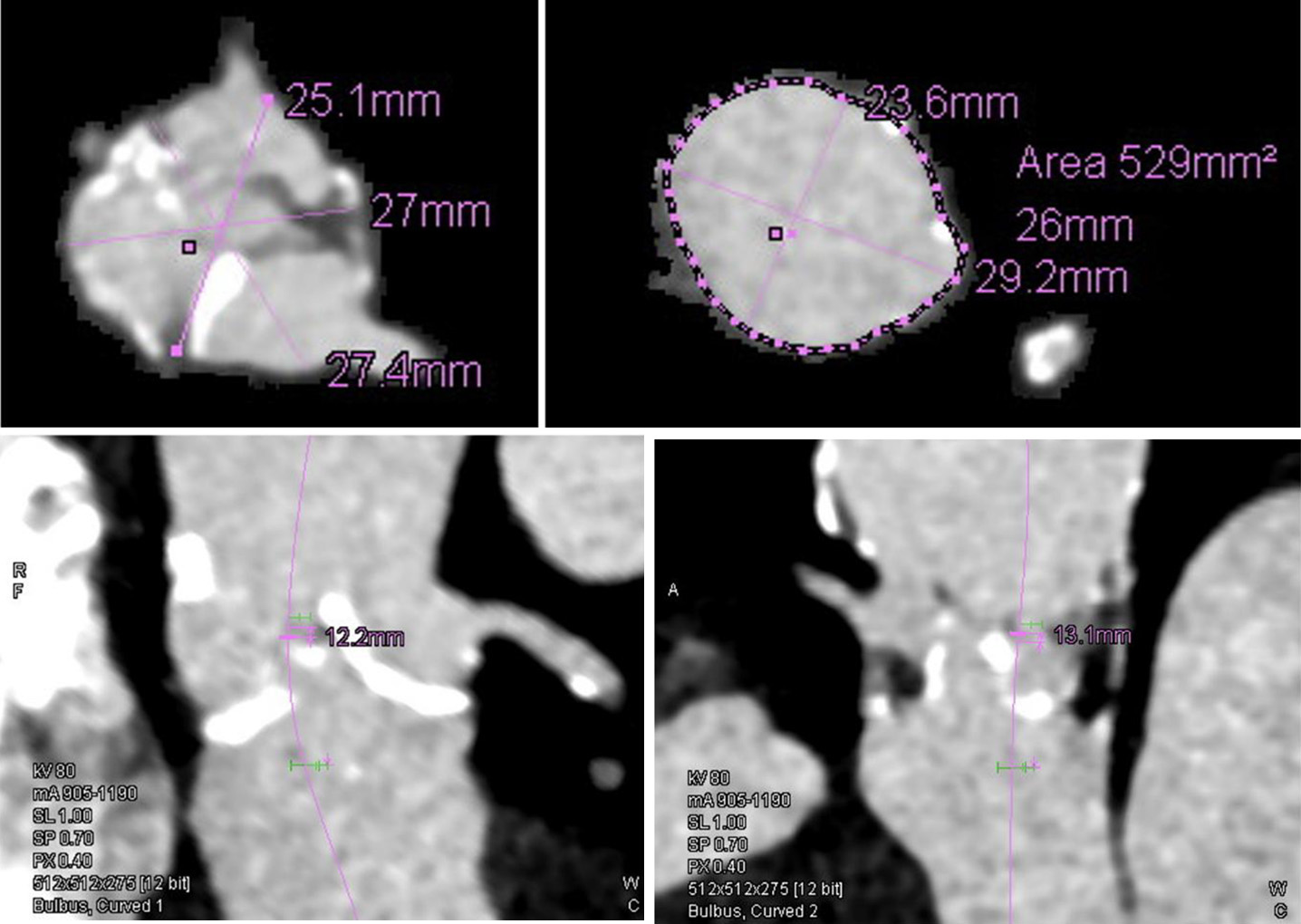 Click for large image | Figure 2. CT aortogram measurements: aortic annulus (up left); sinus of Valsalva (up right); left coronary ostia height (bottom left); right coronary ostia height (bottom right). CT: computed tomography. |
Treatment
Bifemoral approach was obtained by positioning a 16-F transcatheter aortic valve introducer in the left femoral artery and a 6-F introducer for a pig-tail catheter in the right femoral artery. The aortic valve was initially crossed with a 0.0035” straight-tip wire that was later exchanged for a 0.0035” Amplatz SS (Boston Scientific, Boston, MA, USA) using an Amplatz left (AL) 1 catheter (Launcher, Medtronic, MN, USA). As coronary artery ostia occlusion was expected, another 6-F introducer with a Judkins left (JL) 4.0 6-F catheter (Launcher, Medtronic, Minnesota, USA) was positioned in the right femoral artery and a 0.0014” guidewire was advanced in the distal part of the left anterior descending (LAD) artery. Valve pre-dilatation with a Zelos PTA 22 × 40 mm balloon (OptiMed, Germany) was performed. Flow through both coronary arteries was present during balloon inflation (Fig. 3a). Next, we positioned an undeployed everolimus drug-eluting stent (3.0 × 20 mm) in the middle segment of the LAD (Fig. 3b) and proceeded with the implantation of a Sapien 3 valve, underinflating the balloon by 2 mL (Fig. 3c). Immediately after valve expansion occlusion of the left coronary artery was noted. The pre-positioned stent was retracted smoothly from the LAD and positioned from the ostium of the left main coronary artery to the ascending aorta establishing sufficient flow using the “chimney technique”. A proximal balloon post-dilatation (POT) of the aortic stent extension was also performed (Fig. 3d). Minutes later, the patient became hemodynamically unstable developing severe hypotension and subsequent cardiac arrest. Bedside TTE revealed a dilated right ventricle with impaired function, without signs of tamponade. During a short resuscitation, an intra-aortic balloon pump was introduced through the right femoral artery. Aortography revealed a well patent left coronary artery and an occlusion of the right coronary artery (RCA) that could not be engaged even after several attempts using different techniques (Fig. 3e). At the end we decided for a bailout intravenous bolus of unfractionated heparin (5,000 IU) and eptifibatide (180 µg/kg). Following this, flow through the RCA restored and the patient stabilized (Fig. 3f). Control aortography demonstrated a good prosthetic valve position with patent coronary vessels and no residual aortic regurgitation.
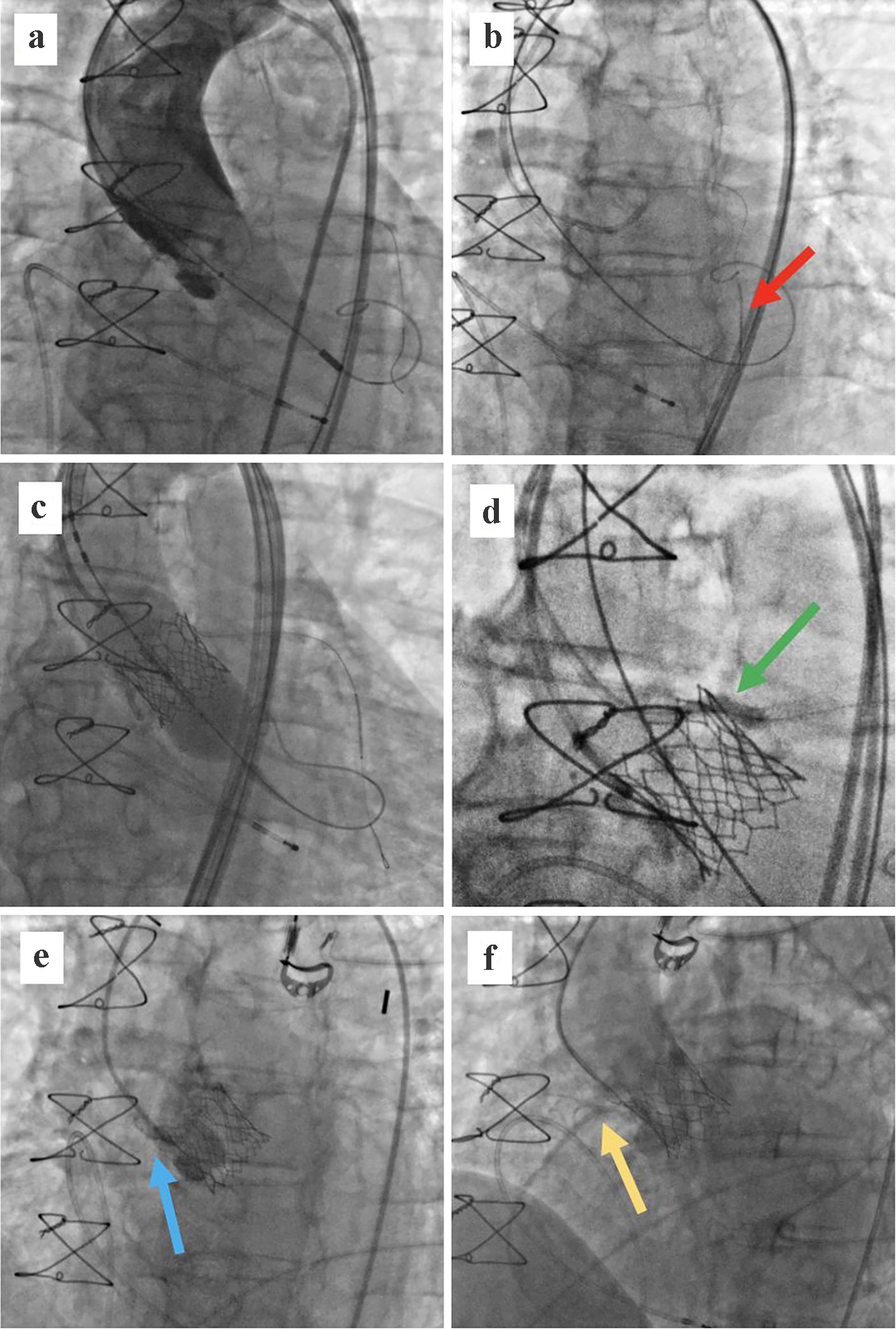 Click for large image | Figure 3. (a) Balloon aortic valvuloplasty with a 22 × 40 mm balloon and a BMW wire placed in the distal LAD. Flow is present in both coronary arteries. (b) Drug-eluted stent placed in the middle segment of the LAD (red arrow). (c) Implantation of a 26-mm balloon-expandable Sapien 3 valve in the annular position. (d) Left main stenting up to the ascending aorta using the “chimney technique”; post-dilatation balloon (green arrow). (e) Aortography showing an occlusion of the RCA (blue arrow). (f) Flow restoration in the RCA after administration of unfractionated heparin and eptifibatide (yellow arrow). LAD: left anterior descending; RCA: right coronary artery. |
Follow-up and outcomes
Following the procedure, patient was maintained on vasoactive and inotropic support for 2 more days in the intensive care unit. Hospitalization was further prolonged by a spontaneous small subarachnoid hemorrhage and subdural hematoma 2 days after procedure that were treated conservatively. Ticagrelor therapy was withheld due to bleeding complications, leaving the patient on only acetylsalicylic acid for 5 days. After documented hematoma resorption and stabilization, dual antiplatelet therapy with clopidogrel was initiated and advised to be continued for further 6 months. Control TTE before discharge showed a mildly decreased left ventricular ejection fraction with an akinetic basal inferior and infero-septal segment, a dilated right ventricle with impaired systolic function (fractional area change of 22%) and a prosthetic aortic valve with mildly elevated doppler signals (maximal velocity of 2.5 m/s, mean gradient of 15 mm Hg) (Fig. 4). With rehabilitation the patient’s cognitive and physical function substantially improved over the next weeks and was discharged after 30 days of hospitalization.
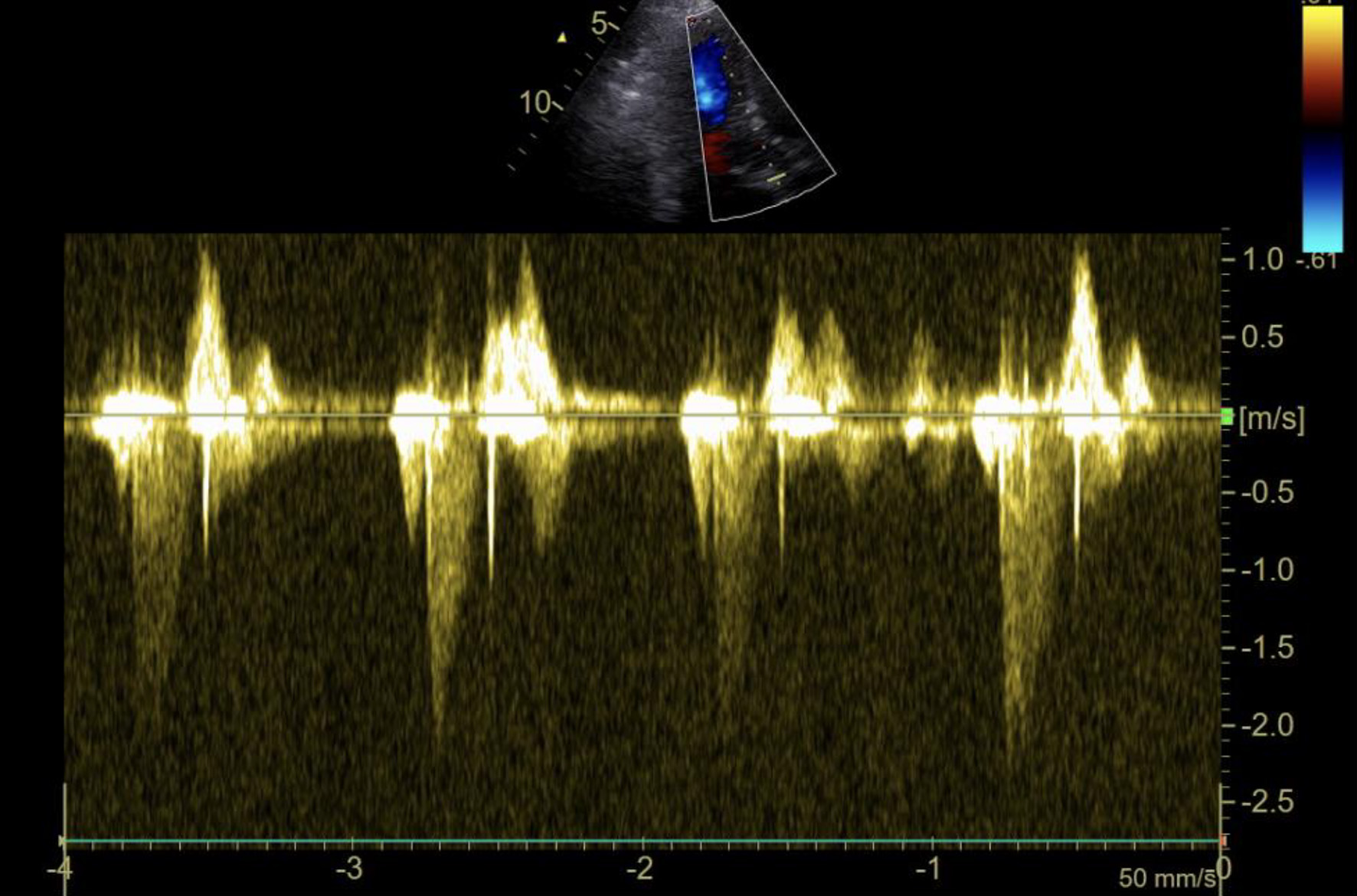 Click for large image | Figure 4. TTE Doppler gradient through the Sapien 3 valve before discharge showing mildly elevated velocity and gradients. TTE: transthoracic echocardiography. |
| Discussion | ▴Top |
As presented by our case report, ViV TAVI in degenerated surgical bioprosthetic valves can be an extremely challenging and complex interventional procedure. With good pre-procedural evaluation, serious complications can be predicted and treatment strategies accordingly adjusted. Our patient had several risk factors for possible ACO during implantation including a small sinus of Valsalva, relatively low coronary ostia and presence of a stentless surgical valve. As it has been shown that left coronary artery is affected by ACO in the vast majority of cases [3], we planned to protect it using the “chimney technique”. This technique creates a stented channel allowing sufficient flow and perfusion in case of ACO. It is a known bailout procedure during TAVI, yet its use is relatively infrequent accounting for 0.5-2.2% of all cases [7-9]. Studies have shown that when anticipated, it lowers the risk of major cardiovascular complications [7]. Recently, another preventive method with intentional endovascular leaflet laceration (BASILICA) has also been described with promising results [10]. In our case, the use of the “chimney technique” allowed us to successfully resolve the ACO of the left coronary artery.
Unfortunately, our patient also had an ACO of the unprotected RCA that led to acute right ventricular failure and cardiac arrest. In such cases difficult coronary ostia engagement and revascularization leads to higher rates of in-hospital morbidity and mortality [7, 11]. Alternative treatments include urgent surgical coronary artery bypass grafting or valve removal using a snare [12, 13]. We tried to gain some hemodynamic support by using an intra-aortic balloon pump, although use of veno-arterial extracorporeal membrane oxygenation has also been reported [11]. Following multiple failed catheter engagement attempts, we decided to infuse an intravenous bolus of unfractionated heparin and eptifibatide. Dosing was calculated according to the ESC guidelines on myocardial revascularization [14]. This proved to be the saving bailout strategy as flow through the RCA restored. We hypothesize that ACO of the RCA was caused by calcific debris from the degenerated aortic valve dislodged by the procedure and turbulent flow between the bioprosthetic valve and aortic wall, leading to possible thrombus formation and coronary artery embolization. To date, no such complication and bailout strategy have been described.
Due to left main coronary stenting and high risk of in-stent thrombosis, we decided for a dual antiplatelet therapy with ticagrelor and acetylsalicylic acid. However, owing to major intra-cranial bleeding complication, we first decided to withhold dual antiplatelet therapy and then use a combination of acetylsalicylic acid and clopidogrel for a shorter duration of 6 months. All decisions were made in concordance with the ESC guidelines on dual antiplatelet therapy [15]. Despite all complications, the patient recovered well and was safely discharged home.
Learning points
With the current rise of ViV TAVI, it is important to consider all possible complications that can arise during the procedure. Here we presented a successful utilization of two different techniques for managing ACO. The “chimney technique” is a useful option but must be prepared in advanced. On the contrary if coronary arteries cannot be engaged with a catheter after implantation, we recommend a treatment attempt with intravenous anticoagulant and antithrombotic therapy if not contraindicated.
Conclusion
ACO after ViV TAVI is a rare but potentially fatal complication associated with poor outcomes. To predict an impending occlusion during TAVI, a variety of different valve, anatomical and physiological factors need to be considered and understood. Upfront coronary artery protection using the “chimney technique” can be a feasible and safe option in case of ACO. However, long-term data about its durability and efficacy are still lacking. In case of unprotected ACO and unsuccessful catheter engagement, use of intravenous anticoagulant and antithrombotic therapy can also be considered as a bailout strategy.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of interest
Authors declare no conflict of interest regarding the publication of this article.
Informed Consent
Patient gave informed consent for the publication of this article including relevant images.
Author Contributions
MB performed the procedure, made substantial contributions to conception, design, and analysis and interpretation of data, and wrote the manuscript. LV wrote and reviewed the manuscript. GPU was involved in the design and interpretation of data and reviewed the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ViV: valve-in-valve; TAVI: transcatheter aortic valve implantation; ACO: acute coronary occlusion; TTE: transthoracic echocardiography; LAD: left anterior descending; RCA: right coronary artery
| References | ▴Top |
- Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312(2):162-170.
doi pubmed - Dvir D, Webb J, Brecker S, Bleiziffer S, Hildick-Smith D, Colombo A, Descoutures F, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012;126(19):2335-2344.
doi pubmed - Ribeiro HB, Rodes-Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, Makkar R, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018;39(8):687-695.
doi pubmed - Rosseel L, Rosseel M, Hynes B, Bel XA, Crilly E, Mylotte D. Chimney Stenting During Transcatheter Aortic Valve Implantation. Interv Cardiol. 2020;15:e09.
doi pubmed - Cockburn J, Dooley M, Parker J, Hill A, Hutchinson N, de Belder A, Trivedi U, et al. Transcatheter aortic valve-in-valve treatment of degenerative stentless supra-annular Freedom Solo valves: A single centre experience. Catheter Cardiovasc Interv. 2017;89(3):438-444.
doi pubmed - Butchart EG, Gohlke-Barwolf C, Antunes MJ, Tornos P, De Caterina R, Cormier B, Prendergast B, et al. Recommendations for the management of patients after heart valve surgery. Eur Heart J. 2005;26(22):2463-2471.
doi pubmed - Mercanti F, Rosseel L, Neylon A, Bagur R, Sinning JM, Nickenig G, Grube E, et al. Chimney stenting for coronary occlusion during TAVR: insights from the chimney registry. JACC Cardiovasc Interv. 2020;13(6):751-761.
doi pubmed - Palmerini T, Chakravarty T, Saia F, Bruno AG, Bacchi-Reggiani ML, Marrozzini C, Patel C, et al. Coronary Protection to Prevent Coronary Obstruction During TAVR: A Multicenter International Registry. JACC Cardiovasc Interv. 2020;13(6):739-747.
doi pubmed - Yamamoto M, Shimura T, Kano S, Kagase A, Kodama A, Koyama Y, Watanabe Y, et al. Impact of preparatory coronary protection in patients at high anatomical risk of acute coronary obstruction during transcatheter aortic valve implantation. Int J Cardiol. 2016;217:58-63.
doi pubmed - Westermann D, Ludwig S, Kalbacher D, Spink C, Linder M, Bhadra OD, Nikorowitsch J, et al. Prevention of coronary obstruction in patients at risk undergoing transcatheter aortic valve implantation: the Hamburg BASILICA experience. Clin Res Cardiol. 2021;110(12):1900-1911.
doi pubmed - Joshi U, Duvvuri P, Barzallo M, Mungee S. Acute ostial right coronary artery occlusion during valve deployment of transcatheter aortic valve replacement leading to acute right ventricular failure: a perfect storm and successful navigation. Cureus. 2020;12(12):e12373.
doi - Maggio S, Gambaro A, Scarsini R, Ribichini F. Preventive left main and right coronary artery stenting to avoid coronary ostia occlusion in high-risk stentless valve-in-valve transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg. 2017;25(1):147-149.
doi pubmed - Fede A, Romano M, Buffoli F, Camurri N, Lettieri C. Combination of double chimney technique and prosthesis post-dilation after valve-in-valve implantation. JACC Case Rep. 2020;2(14):2173-2175.
doi pubmed - Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2019;55(1):4-90.
doi pubmed - Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


