| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 3, March 2022, pages 99-103
Gemella morbillorum as the Culprit Organism of Post-Colonoscopy Necrotizing Perineal Soft Tissue Infection in a Diabetic Patient With Crohn’s Disease
Eltaib Saada, c, Apoorva Tummalab, Mohamed Agaba, Guillermo Rodriguez-Navaa
aDepartment of Internal Medicine, Saint Francis Presence Hospital, Evanston, IL, USA
bSchool of Medicine, the University of Illinois at Chicago, Chicago, IL, USA
cCorresponding Author: Eltaib Saad, Department of Internal Medicine, Saint Francis Presence Hospital, Evanston, IL, USA
Manuscript submitted January 3, 2022, accepted January 27, 2022, published online March 5, 2022
Short title: G. morbillorum and Soft Tissue Infection
doi: https://doi.org/10.14740/jmc3896
| Abstract | ▴Top |
Gemellsa morbillorum (G. morbillorum) is a Gram-positive facultative anaerobe and a known commensal organism of the oropharyngeal and gastrointestinal tracts. It is considered a rare cause of infections in humans. Most of the documented infections, whereas G. morbillorum has been implicated as a causative pathogen, were infective endocarditis and deep visceral abscesses. However, there are only a handful of cases in the current literature that have reported G. morbillorum as the primary organism causing necrotizing soft tissue infections. The authors presented a rare case of post-colonoscopy necrotizing perineal soft tissue infections in an elderly patient with poorly controlled diabetes mellitus and Crohn’s disease with G. morbillorum being the culprit pathogen of this necrotizing infection. The reported case raises concerns for this commensal organism as an emerging virulent pathogen in certain high-risk patients. The authors proposed that a combination of the long-standing Crohn’s disease and the recent colonoscopy with rectal polypectomy has predisposed the patient to G. morbillorum bacteremia with perineal sepsis in the setting of diabetic immunosuppression. Further studies are warranted to ascertain whether G. morbillorum is acquiring increased virulence that would have enabled this organism to cause novel soft tissue infections.
Keywords: G. morbillorum; Rare pathogen; Bacteremia; Diabetic immunosuppression; Crohn’s disease; Colonoscopy; Necrotizing soft tissue infection
| Introduction | ▴Top |
Gemella morbillorum (G. morbillorum) is a Gram-positive facultative anaerobe and a known commensal organism of the oropharyngeal and gastrointestinal tracts [1, 2]. It is considered a rare cause of infections in humans [3]. Most of the reported infections, whereas G. morbillorum has been isolated as a causative pathogen, were infective endocarditis, septic arthritis, and deep visceral abscesses [4-10]. However, there are only a handful of cases in the current literature that has documented G. morbillorum as a primary organism causing necrotizing soft tissue infections [3]. In this report, we present a rare case of a necrotizing perineal soft tissue infection caused by G. morbillorum in an elderly diabetic female patient with a history of long-standing Crohn’s disease who underwent a recent colonoscopy with rectal polypectomy. This case highlights G. morbillorum as the culprit organism of necrotizing perineal soft tissue infections and raises concerns for this commensal organism as an emerging virulent pathogen in high-risk patients.
| Case Report | ▴Top |
Investigations
An 87-year-old Caucasian female patient was brought to the emergency department with recurrent fevers and worsening perineal pain for 2 weeks. She denied recent bowel movements changes, bloody diarrhea, abdominal pain, vomiting, or melena. She had no cough or shortness of breath. The patient denied any urinary symptoms. The systemic review was not contributing to her presenting complaints. Past medical history was significant for poorly controlled type II diabetes mellitus, Crohn’s disease diagnosed 18 years ago, status post-right hemicolectomy for perforated ileocolic disease, chronic secretory diarrhea secondary to bile acids malabsorption, gastroesophageal reflux disease, and hypertension. Her regular medications included mesalamine, metformin, glipizide, amlodipine, and omeprazole. Notably, the patient underwent a recent surveillance colonoscopy performed 3 weeks prior to her presentation, which was largely unremarkable apart from a small hyperplastic polyp in the lower rectum that was removed with a cold snare. Serial random colonic and ileocolic anastomosis biopsies were negative for any dysplastic or neoplastic changes.
Physical examination revealed an ill-appearing patient who was febrile to 38.8 °C, with a pulse rate of 110 beats per minute and blood pressure of 95/65 mm Hg. The abdomen was soft and non-tender to examine. The perineal examination was remarkable for a necrotic-looking blackish eschar over the left ischioanal region with profound cellulitis changes. No palpable crepitus or bullae were evident. Digital rectal examination revealed no internal rectal induration. The systemic examination was unremarkable.
Diagnosis
Laboratory findings showed a white blood cell count of 17.5 × 109/L (reference 3.0 - 11.0 × 109/L), C-reactive protein (CRP) of 75 mg/dL (reference 1.5 - 7.0 mg/dL), hyponatremia of 128 mmol/L (reference 133 - 144 mmol/L), hypokalemia of 3.4 mmol/L (reference 3.5 - 5.0 mmol/L), and a normal serum creatinine level. Serial blood glucose levels were averaging 350 mg/dL (reference 70 - 110 mg/dL) with a hemoglobin A1c of 9.0% (normal < 5.7%). Computed tomography (CT) scan of the pelvis revealed soft tissue gas within the left ischiorectal fossa with no evident local fluid collection or abscess formation (Fig. 1). There were neither signs of rectal perforation nor fistulous communication. In addition, there were no features of active Crohn’s colitis. The extra-luminal gas was presumed as a result of a necrotizing perineal soft tissue infection. The patient was commenced on broad-spectrum antibiotics (piperacillin/tazobactam, vancomycin, and clindamycin) as per hospital-based guidelines for necrotizing soft tissue infections that were generated from local antibiotics susceptibility patterns. The patient underwent an emergent debridement of the perineal necrotic tissues to a base of viable tissues. There was no evidence of a visible communication of the deep wound cavity with the rectal wall. An intraoperative flexible sigmoidoscopy revealed a grossly normal rectal mucosa.
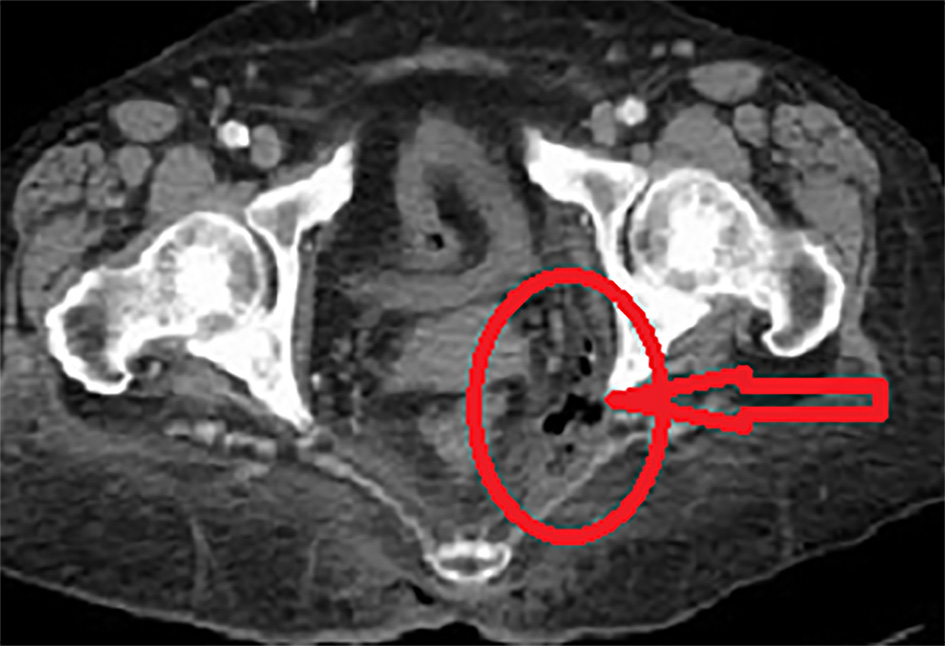 Click for large image | Figure 1. A contrast-enhanced pelvic CT scan demonstrated left ischiorectal fossa infection with extra-luminal soft tissue gas (red circle and red arrow) concerning for a necrotizing soft tissue infection. No signs of rectal perforation or fistulous communication. CT: computed tomography. |
Two sets of blood cultures that were obtained at the time of admission grew Gram-positive cocci in chains within the first day of incubation. Subsequent cultures grew only G. morbillorum that was confirmed by direct matrix-associated laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry from the positive blood culture bottles. There was no growth noted in the aerobic bottles after 5 days of standard incubation. Considering G. morbillorum bacteremia, echocardiography was warranted to rule out concurrent infective endocarditis, and a transesophageal echocardiogram (TEE) was negative for valvular vegetations. The deep perineal wound cultures also grew G. morbillorum.
Treatment
The patient underwent a series of daily wound dressings, and a vacuum-assisted closure (VAC) dressing was applied in situ to enhance wound healing. The patient remained afebrile for 3 days post-debridement with continued parenteral broad-spectrum antibiotics. Repeat blood cultures on day 4 postoperatively were negative for any bacterial growth. The isolated strain of G. morbillorum was pan-sensitive to most antibiotics, except metronidazole. The patient was transitioned to oral clindamycin and amoxicillin as per susceptibility results with a plan to complete at least 2 weeks of antimicrobial therapy as per infectious diseases consultation. Optimization of glycemic control was achieved.
Follow-up and outcomes
The patient was eventually discharged on day 12 post-operatively into a skilled nursing facility for wound care. A 4-week follow-up at an outpatient clinic revealed a well-healing wound.
| Discussion | ▴Top |
G. morbillorum is a part of the commensals of the oropharyngeal and gastrointestinal tracts [1, 2]. It was first described as Streptococcus morbillorum, then Peptostreptococcus morbillorum [2], and then it was eventually classified to the current genus in 1988 based on 16S RNA analysis [2]. G. morbillorum has been more frequently isolated in recent years [5, 7] owing to advances in laboratory detection techniques such as MALDI-TOF, which was employed in our patient, polymerase chain reaction (PCR), particularly in the setting of culture-negative infections [5], and sequence analysis of 16S RNA [7].
Human infections due to G. morbillorum are an uncommon occurrence, but they have been well described in the literature with certain predisposing factors [4-7]. The most reported infections were infective endocarditis, affecting both native and prosthetic valves, which were usually encountered in the setting of post-endoscopic G. morbillorum bacteremia, poor dental hygiene, various immunosuppression states (such as diabetes mellitus and steroids use), and intravenous drug use [4-6], pleural empyema, which was likely related to possible aspiration of oropharyngeal contents [7], septic arthritis [8], spondylodiscitis [9], brain abscess [10], retropharyngeal abscess [11], lung abscess [1], and mediastinitis with mediastinal abscess complicating mycotic thoracic aortic aneurysm [12].
Nevertheless, necrotizing soft tissue infections due to G. morbillorum are rather a rare entity [3, 13-15], as per our literature review of the available English medical literature; only five cases of soft tissue infections were identified where G. morbillorum being isolated as a causative pathogen [3, 13-15]. Table 1 summarizes the characteristics of these cases. It is worth mentioning that G. morbillorum is difficult to isolate in cultures [14], which may partially explain the rarity of published cases in the current literature [14].
 Click to view | Table 1. Summary of Characteristics of Reported Cases of Necrotizing Soft Tissue Infections Caused by G. morbillorum |
This reported patient had a multitude of possible risk factors for a necrotizing perineal soft tissue infection by a commensal gastrointestinal organism such as G. morbillorum. Poorly controlled diabetes mellitus is a well-documented immunosuppressive condition that is associated with significantly reduced clearance of bacteremia and delayed wound healing [8]. A long-standing Crohn’s disease has certainly posed a risk for G. morbillorum bacteremia in this patient, as it has been observed that patients with inflammatory bowel diseases (IBD) are generally at significantly higher risks of gastrointestinal organisms’ bacteremia than the general population [16], this risk may be explained by many postulations that include, for instance, alterations of the gut barrier function of the inflamed intestinal epithelia, which leads to increased mucosal permeability and subsequently bacterial translocation, malnutrition-associated immune dysfunction, and surgically related complications such as colonic perforation and fistulas formation [16]. Furthermore, age was found to be an independent risk of IBD-associated bacteremia, as a recent large retrospective study published by Goren et al reported that 1.3% of hospitalized IBD patients had bacteremia; those patients were mostly above the age of 65 years [16], in keeping with our reported patient’s characteristics.
In addition, the recent colonoscopy with rectal polypectomy may have also contributed to G. morbillorum-associated necrotizing perineal infection in the reported patient. Although there were neither features of a rectal perforation nor fistulous communication on both contrast-based CT imaging and intraoperative sigmoidoscopy, a possibility of micro-perforation cannot be entirely ruled out, and alternatively a subtle rectal perforation could have occurred, resulting in G. morbillorum translocation to the perirectal soft tissues in the setting of diabetic immunosuppression. The latter assumption is supported by the isolation of G. morbillorum from the deep perineal wound cultures.
Interestingly, G. morbillorum bacteremia has been a culprit for a diagnosis of an underlying colonic adenocarcinoma [17, 18]. The association between colonic cancers and certain gastrointestinal organisms’ bacteremia (S. milleri, S. agalactiae, group G streptococci, and Clostridium septicum) is well known [17, 18]. This association could be attributed to a tumor-induced disruption of the gut barrier that potentiates the translocation of colonic bacterial flora [18]. Some authors presently advocate for screening for colonic adenocarcinoma in patients with G. morbillorum bacteremia of unknown source [18]. Notably, our patient’s recent surveillance colonoscopy was negative for neoplastic lesions in the setting of a long-standing Crohn’s disease.
Most isolated G. morbillorum species in the literature were generally sensitive to most antibiotics, but metronidazole [12-14], in line with the strain isolated in our patient. At present, penicillin G and ampicillin are considered of choice for extra-abdominal Gemella infections [19]. However, resistance to penicillin has been increasingly reported in recent years [19]. Most reported cases of G. morbillorum endocarditis responded to a 4- to 6-week course of a combination of penicillins and aminoglycosides [20]. In penicillin-allergic patients or those with penicillin-resistant strains, vancomycin as monotherapy or in combination with erythromycin or rifampicin has been employed with a satisfactory outcome [4, 20].
Learning points
Our case provides evidence of G. morbillorum as the culprit organism of a post-colonoscopy necrotizing perineal soft tissue infection in a diabetic patient with Crohn’s disease.
The reported case raises concerns of this commensal organism as an emerging virulent pathogen in high-risk patients.
The authors proposed that a combination of a long-standing Crohn’s disease and recent colonoscopy with rectal polypectomy could have predisposed our patient to G. morbillorum bacteremia with perineal sepsis in the setting of diabetic immunosuppression.
Further studies are warranted to ascertain whether G. morbillorum is acquiring increased virulence that would lead to novel pathogenesis of soft tissue infections.
Acknowledgments
The authors would like to acknowledge the Department of Infectious Diseases at Saint Francis Presence Hospital for providing valuable input to the critical review of this case presentation.
Financial Disclosure
The authors confirm that there is no funding to declare regarding the publication of this case report.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this report.
Informed Consent
Informed written consent was obtained from the patient to write and publish their case as a case report with all accompanying clinical and radiological images. No identifying persons identifying information has been used in this article.
Authors Contributions
ES and AT contributed equally to the conceptualization and writing the first manuscript. MA and GR have performed the critical review and editing of the final draft. All authors were involved in the clinical management of the reported patients. All authors agreed to the final draft submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- da Costa CT, Porter C, Parry K, Morris A, Quoraishi AH. Empyema thoracis and lung abscess due to Gemella morbillorum. Eur J Clin Microbiol Infect Dis. 1996;15(1):75-77.
doi pubmed - Kilpper-Balz R, Schleifer KH. Transfer of Streptococcus morbillorum to the Genus Gemella as Gemella morbillorum comb. nov. International Journal of Systematic and Evolutionary Microbiology. 1988;38(4):442-443.
doi - Ueberroth BE, Roxas R. Gemella morbillorum isolated from a pelvic abscess in an HIV-positive patient with squamous cell carcinoma of the perianal region. BMJ Case Rep. 2019;12(5):e227352.
doi pubmed - Ural S, Gul Yurtsever S, Ormen B, Turker N, Kaptan F, El S, Akyildiz ZI, et al. Gemella morbillorum Endocarditis. Case Rep Infect Dis. 2014;2014:456471.
doi pubmed - Ramanathan A, Gordon SM, Shrestha NK. A case series of patients with Gemella endocarditis. Diagn Microbiol Infect Dis. 2020;97(1):115009.
doi pubmed - Akiyama K, Taniyasu N, Hirota J, Iba Y, Maisawa K. Recurrent aortic valve endocarditis caused by Gemella morbillorum—report of a case and review of the literature. Jpn Circ J. 2001;65(11):997-1000.
doi pubmed - Yamakawa H, Hayashi M, Tanaka K, Kuwano K. Empyema due to Gemella morbillorum is diagnosed by 16S ribosomal RNA gene sequencing and a phylogenetic tree analysis: a case report and literature review. Intern Med. 2015;54(17):2231-2234.
doi pubmed - Omran Y, Wood CA. Endovascular infection and septic arthritis caused by Gemella morbillorum. Diagn Microbiol Infect Dis. 1993;16(2):131-134.
doi - Sono T, Takemoto M, Shinohara K, Tsuchido Y. An Uncommon Case of Pyogenic Spondylodiscitis Caused by Gemella morbillorum. Case Rep Orthop. 2018;2018:3127613.
doi pubmed - Chotai S, Moon HJ, Kim JH, Kim JH, Chung HS, Park YK, Kwon TH. Brain abscess caused by Gemella morbillorum: case report and review of the literature. Turk Neurosurg. 2012;22(3):374-377.
- Pradeep R, Ali M, Encarnacion CF. Retropharyngeal abscess due to Gemella morbillorum. Clin Infect Dis. 1997;24(2):284-285.
doi pubmed - Said M, Tirthani E. Gemella morbillorum- and Capnocytophaga sp.-related mycotic thoracic aortic aneurysm and mediastinal abscess: an unusual case report, a treatment challenge, and a review of literature. Cureus. 2021;13(9):e17728.
doi - Bachmeyer C, Landgraf N, Daumas L. Soft tissue infection caused by Gemella morbillorum in two intravenous drug users. J Am Acad Dermatol. 2005;52(4):704-705.
doi pubmed - Romero-Velez G, Pereira X, Narula A, Kim PK. Gemella morbillorum as a source bacteria for necrotising fasciitis of the torso. BMJ Case Rep. 2020;13(1):e231727.
doi pubmed - Rosina P, Cunego S, Meloni G, Favari F, Leoni A. Cutaneous and systemic infection by Gemella morbillorum. Acta Derm Venereol. 1999;79(5):398.
doi pubmed - Goren I, Brom A, Yanai H, Dagan A, Segal G, Israel A. Risk of bacteremia in hospitalised patients with inflammatory bowel disease: a 9-year cohort study. United European Gastroenterol J. 2020;8(2):195-203.
doi pubmed - FitzGerald SF, Moloney AC, Maurer BJ, Hall WW. Gemella endocarditis: consider the colon. J Heart Valve Dis. 2006;15(6):833-835.
- Reyes R, 3rd, Abay A, Siegel M. Gemella morbillorum bacteremia associated with adenocarcinoma of the cecum. Am J Med. 2001;111(2):164-165.
doi - Garcia-Lechuz JM, Cuevas-Lobato O, Hernangomez S, Hermida A, Guinea J, Marin M, Pelaez T, et al. Extra-abdominal infections due to Gemella species. Int J Infect Dis. 2002;6(1):78-82.
doi - Kofteridis DP, Anastasopoulos T, Panagiotakis S, Kontopodis E, Samonis G. Endocarditis caused by Gemella morbillorum resistant to beta-lactams and aminoglycosides. Scand J Infect Dis. 2006;38(11-12):1125-1127.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


