| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 3, March 2022, pages 135-139
Tocilizumab-Associated Small Bowel Perforation in a Young Patient With Rheumatoid Arthritis: A Lesson to Remember During COVID-19 Pandemic
Eltaib Saada, c, Abdalaziz Awadelkarimb, Mohamed Agaba, Akram Babkira
aDepartment of Internal Medicine, AMITA Saint Francis Hospital, Evanston, IL, USA
bDepartment of Internal Medicine, Detroit Medical Center/Wayne State University, Detroit, MI, USA
cCorresponding Author: Eltiab Saad, Department of Internal Medicine, Saint Francis Presence Hospital, Evanston, IL, USA
Manuscript submitted January 14, 2022, accepted February 18, 2022, published online March 5, 2022
Short title: Tocilizumab-Associated Small Bowel Perforation
doi: https://doi.org/10.14740/jmc3902
| Abstract | ▴Top |
Tocilizumab is a recombinant humanized monoclonal antibody directed against the interleukin-6 (IL-6) receptor, which has been used for the treatment of rheumatoid arthritis (RA). A range of side effects have been associated with tocilizumab, with gastrointestinal perforation (GIP) being described as a rare but potentially life-threatening complication that deserves considerable attention. The authors report a case of a young male patient with a history of challenging RA who encountered a lower GIP that was associated with tocilizumab therapy. The occurrence of tocilizumab-induced GIP in this reported patient had initially posed a diagnostic dilemma, as its clinical presentation mimicked other autoimmune inflammatory and infectious diseases that are commonly associated with RA. Physicians should be aware of GIPs as a serious adverse event of tocilizumab use despite being a rare phenomenon, particularly in the era of the global pandemic of coronavirus disease 2019 (COVID-19), when this novel drug has been authorized for the management of selected patients with severe COVID-19 infection. Therefore, early recognition and timely management of GIPs would minimize potential morbidities associated with critically ill COVID-19 patients.
Keywords: Tocilizumab; Gastrointestinal perforations; Rare; Rheumatoid arthritis; COVID-19
| Introduction | ▴Top |
Tocilizumab is a humanized interleukin-6 (IL-6) monoclonal antibody that targets both membrane-bound and soluble forms of IL-6 receptors, thus decreasing signal transduction through glycoprotein-130 (gp130) with varied downstream effects on both innate and adaptive immunity [1]. It has been approved for the treatment of selected patients with rheumatoid arthritis (RA) in the United States of America since 2010 [2]. Common side effects include oral ulcers, immunosuppression, anaphylactic reaction, and hepatotoxicity [1-4]. Nevertheless, gastrointestinal perforations (GIPs) were recently reported as a rare but potentially life-threatening adverse effect of tocilizumab therapy which warrants serious attention [2, 3]. The current estimated incidence of tocilizumab-associated GIP is approximately 2.7 - 3.0/1,000 patient-years [2, 3]. RA is associated with a range of gastrointestinal manifestations related to various etiologies (such as direct RA effects, sequelae of immunosuppressive treatment, and associated autoimmune diseases) [4]. The authors present a case of a young patient with RA who encountered a tocilizumab-associated lower GIP that necessitated an operative intervention. The available literature is reviewed, and similar cases of GIP associated with tocilizumab during the current coronavirus disease 2019(COVID-19) pandemic were briefly discussed.
| Case Report | ▴Top |
Investigations
A 29-year-old Caucasian male patient with a past medical history of treatment-resistant seronegative RA presented to the emergency department (ED) with severe generalized abdominal pain for 3 days associated with nausea, bilious vomiting, and watery diarrhea. The pain has been ongoing for the last 3 weeks prior to the index presentation with less severity and was described as colicky upper and mid-abdominal associated with nausea and loose bowel movements. The patient denied mucous or bloody diarrhea, rectal bleeding, melena, or hematemesis. No history of reflux symptoms was elicited. He denied fevers or rigors, weight loss, jaundice, anorexia, or skin rash. The patient had no history of sick contacts or recent antibiotic use. The systemic review was negative for other pertinent symptoms. No previous similar presentations were encountered.
The patient was diagnosed with RA 6 years prior to presentation; he tried many disease-modifying antirheumatic treatments (DMARTs) and biological agents with poor clinical response and continued challenging symptoms. The patient did not tolerate benepali (etanercept), baricitinib was discontinued following an unprovoked episode of superficial phlebitis, and adalimumab was associated with severe skin rash. Finally, tocilizumab (162 mg, weekly injection) was commenced 2 weeks prior to the onset of presenting symptoms. Notably, the last dose of tocilizumab was administered 5 days before the current presentation. The regular medications included prednisolone 5 mg daily, omeprazole 20 mg daily, methotrexate 20 mg once weekly, and folic acid 5 mg daily. He had no family history of inflammatory bowel disease (IBD).
One week before the index presentation to the ED, the patient attended an outpatient gastroenterology clinic for evaluation. Complete blood count (CBC) and comprehensive metabolic profile (CMP) were within the normal limits, serum antibodies for the coeliac disease were undetectable, and a stool panel for bacteria and parasites did not yield any infectious culprit. He underwent esophagogastroscopy (EGD) and ileo-colonoscopy. EGD was entirely unremarkable, and particularly no features of erosive gastritis were visualized given the current steroids use. Ileo-colonoscopy showed only mild inflammatory changes at the distal ileum with a grossly normal colon. Ileal biopsies revealed non-specific inflammation without characteristic features for IBD. The result of colonic biopsies for cytomegalovirus (CMV) was negative in the context of concurrent immunosuppressive therapy.
On initial evaluation at the ED, the patient was afebrile with stable vital signs. Abdominal examination revealed generalized abdominal tenderness without signs of peritonitis. The rest of the systemic examination was unremarkable.
Diagnosis
Laboratory workup showed leukocytosis (white cell count (WCC) of 14,000/mm3 with reference range 3,000 - 11,000/mm3) and elevated C-reactive protein (CRP) of 70 mg/dL (reference range 1.0 - 5.0 mg/dL). The rest of the blood results were unremarkable. Nasopharyngeal screening for COVID-19 was negative. A computed tomography (CT) scan of the abdomen demonstrated dilated small bowel loops centered on the distal ileum with adjacent fat stranding (Fig. 1a, b). There was no bowel obstruction or perforation, and no evidence of diverticular disease was noted.
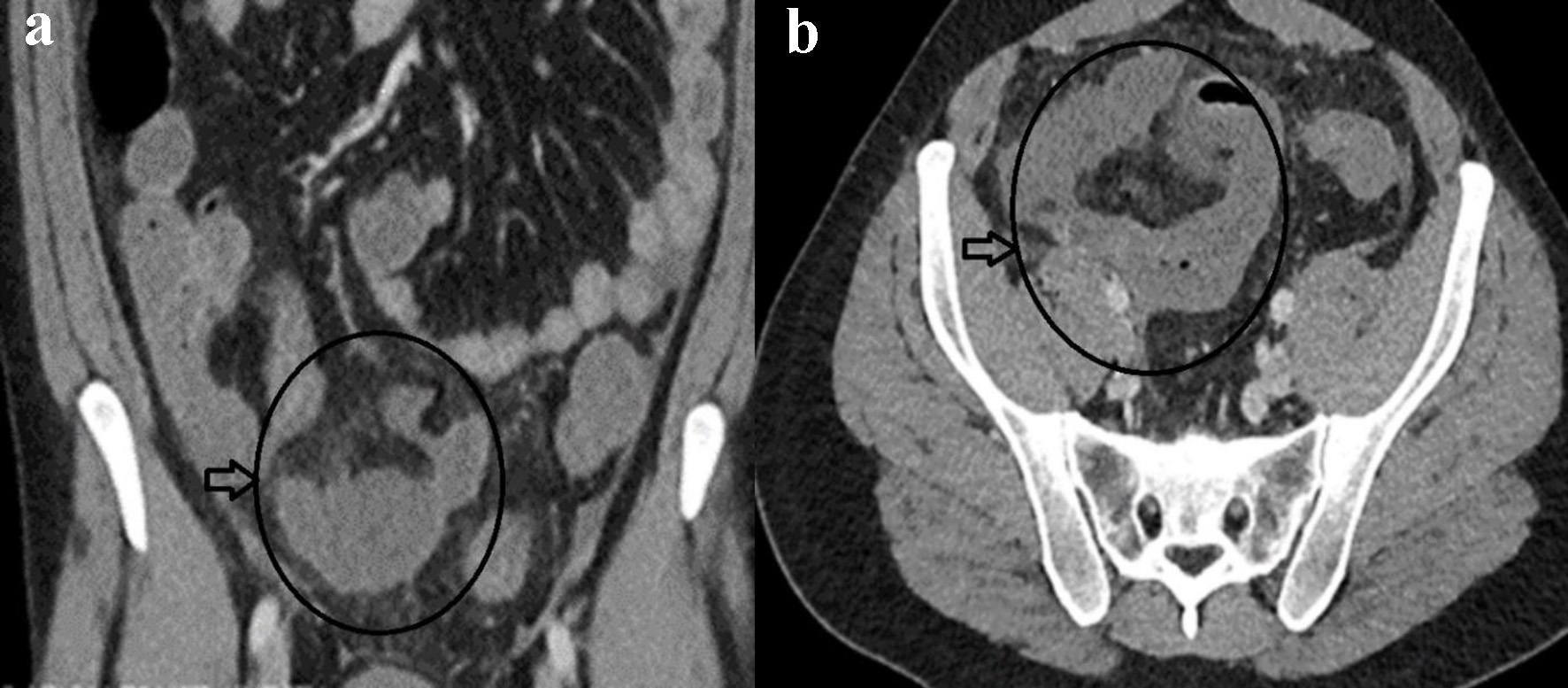 Click for large image | Figure 1. Coronal (a) and axial (b) contrast-enhanced CT of abdomen showing mural dilated small bowel loop mural enhancement at the distal ileum with localized edema (circle and horizontal arrow in a, b). |
The differential diagnosis included IBD as the likely etiology, as well as infectious enteritis and mesenteric ischemia secondary to medium or large-vessel vasculitis. Broad-spectrum antibiotics (piperacillin/tazobactam and gentamicin) were commenced as per hospital-based guidelines for intraabdominal sepsis. Fecal calprotectin levels were not elevated, and stool cultures were negative for bacterial or parasitic growth. The infectious disease team recommended workup for tuberculosis enteritis, considering the recent treatment with biological agents. The patient was tested for latent tuberculous infection with the Quantiferon gold assay, which was non-reactive, and chest CT was unremarkable. Polymerase chain reaction (PCR) for Clostridium difficle was negative. Additionally, serial blood cultures were negative for Gram-negative bacteria, including Salmonella species.
Rheumatology service was consulted given concerns of mesenteric vasculitis in the setting of RA. They advised that the occurrence of vasculitis would be very unusual while the patient was on long-term steroids and methotrexate. Additionally, CT mesenteric angiogram was negative for mesenteric vasculitis. Methotrexate was then held as per rheumatology, and a stress dose of steroids was recommended in the context of sepsis.
On the third day of admission, the patient spiked a high-grade fever (to 38.4 °C). He was tachycardiac to 130 beats per minute and hypotensive to 80/50 mm Hg. Physical examination revealed a peritonitic abdomen. CRP level almost doubled to 135 mg/dL and serum lactate was 3.3 mmol/L (reference range 0.5 - 2.2 mmol/L). Repeat COVID-19 PCR from the nasopharyngeal swab was negative. Repeat abdominal CT scan depicted a localized perforation arising from the distal small bowel with a small free air and fluid mesenteric collection (Fig. 2a, b). Surgical consultation recommended an emergent operative intervention for sepsis source control.
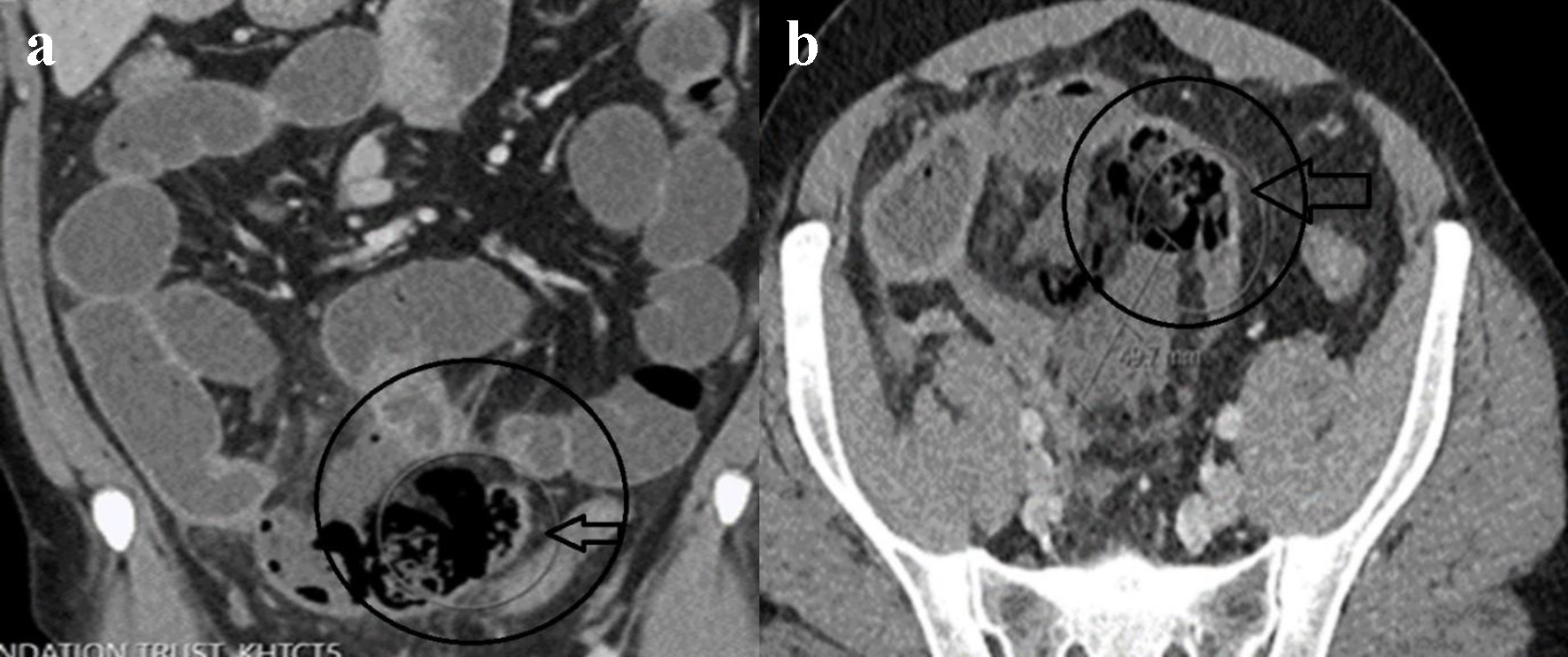 Click for large image | Figure 2. Repeat contrast-enhanced CT of abdomen. Coronal (a) and axial (b) views showing localized distal ileal perforation with free gas locules and contained perforation (circle and horizontal arrow in a, b). |
Treatment
Operative findings revealed a localized distal ileum perforation with full-thickness necrosis of the distal 20 cm of the ileum. Distal small bowel resection was performed, and an end ileostomy and mucous fistula were fashioned. No primary anastomosis was created considering the relatively high risk of anastomotic leak due to significant local sepsis on a background of immunosuppression. Postoperative recovery was uneventful. The histopathology of the resected bowel was unremarkable, with no evidence of IBD, vasculitis, or tuberculous enteritis.
Tocilizumab was considered the likely cause of bowel perforation, as extensive diagnostic workup did not reveal any underlying etiological culprit for the bowel pathology, and then it was discontinued prior to discharge following interdisciplinary discussions.
Follow-up and outcomes
The patient denied recurrence of his presenting symptoms over serial outpatient visits. After 2 months, a magnetic resonance (MR) scan of the small bowel was utterly unremarkable for suspicious bowel lesions. Once again, no radiological features of IBD were demonstrated. A per stoma ileoscopy revealed a grossly normal mucosa of neo-distal ileum. The patient eventually underwent a reversal of ileostomy after 3 months with an uncomplicated postoperative course.
| Discussion | ▴Top |
The reviewed literature has reported that patients with RA may be at particular risk of GIPs due to various causes [1-5]. Historically, upper GIPs were typically more associated with RA due to non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids use [4, 5]. We described a case of lower GIP perforation that was associated with tocilizumab therapy, in line with recent studies that reported that most perforations of tocilizumab occurred in the lower gastrointestinal tract [1-3, 6]. It is well documented at present that lower GIPs are a rare but costly and life-threatening complication of tocilizumab [1-3, 5, 6]. In addition, concomitant use of steroids and the presence of active diverticular disease significantly increase this risk of the lower GIPs in the RA population [1, 3].
The pathophysiology of tocilizumab-induced GIP remains poorly understood [1, 2], but it was theorized that one of the effects of the IL-6 blockade is a decrease of expression of vascular endothelial growth factor (VEGF), which plays an essential role in maintaining the integrity of intestinal mucosa [7].
Tocilizumab is considered the underlying culprit of lower GIP given a strong temporal relationship between tocilizumab use and the onset of gastrointestinal complaints in our patient. The patient started to report his symptoms shortly after the initiation of tocilizumab; at that time, the bowel inflammation was only mild on colonoscopy, but further doses have eventually resulted in frank bowel perforation, possibly due to the accumulative drug effect. The lack of possible infectious, inflammatory, or vascular etiologies to explain the patient’s symptoms also supports this presumptive association. Furthermore, prompt symptoms resolution following discontinuation of tocilizumab with the absence of any radiological or endoscopic evidence of small bowel disease after stopping the drug strongly points to the causal relationship between tocilizumab and the occurrence of lower GIP in our patient.
The bowel perforation had initially posed a diagnostic challenge in this reported patient, as the clinical and radiological findings simulated other common autoimmune and infectious conditions that are related to RA [4]. Interestingly, in a cohort of patients with IBD and matched group, the IBD group had two times higher odds of developing RA as compared to the control [4], suggesting an association between the two autoimmune entities [4]. Nevertheless, there was no endoscopic or histological evidence to prove IBD in our patient. Moreover, it is known that patients with RA treated with biologics are at significant risk of tuberculous enteritis that often presents with a non-specific constellation of symptoms, thus making a diagnostic dilemma in RA patients [4]; hence, it was essential to exclude tuberculous infection with serological and histopathological diagnostics in our patient as the recent immunosuppression may have masked the patient’s cell-mediated immune response to the tuberculous infection.
At the time of the global COVID-19 pandemic, tocilizumab has been increasingly used to treat selected patients with critical COVID-19 infections since the publication of the RECOVERY trial [8]. A recent review has reported that GIPs can be an unusual complication of severe COVID-19 disease, as a total of 25 cases of COVID-associated GIPs were identified up to the time of that review writing in April 2021 [9], with seven of those cases being treated with tocilizumab [9], supporting pre-pandemic literature that documented this association. Understandably, the concomitant use of steroids compounds the GIP risks among the COVID-19 population [9]. It is worth mentioning that GIPs were associated with adverse morbidities in critical COVID-19 patients [9]. Therefore, physicians should be aware of this serious complication of tocilizumab therapy despite being a rare phenomenon, and early recognition with timely management of GIPs would minimize further potential morbidities. Nevertheless, diagnosis of tocilizumab-induced GIPs may be challenging, as some COVID-19 patients may not experience classical signs of intraabdominal sepsis due to immunosuppression with steroids [10]. In addition, CRP levels may not correlate with the severity of sepsis due to tocilizumab’s effects on CRP [9, 10].
Conclusion
Patients with RA are subjected to a particular risk of GIPs due to various causes, with tocilizumab treatment being one of these unusual etiologies, as demonstrated in this reported patient. GIPs are associated with significant morbidity; hence, careful consideration is warranted. Physicians should be aware of this serious complication of tocilizumab use despite being a rare phenomenon, particularly in the era of the global COVID-19 pandemic when this novel drug has been authorized for the management of critical COVID-19 infections. Therefore, early diagnosis and timely management would minimize potential morbidities associated with GIP in critically ill patients.
Learning points
RA patients are subjected to a particular risk of upper and lower GIPs due to various etiologies.
Lower GIPs are a rare but costly and life-threatening complication of tocilizumab use.
Physicians should be aware of this serious complication of tocilizumab use despite being a rare phenomenon, particularly in the era of the global COVID-19 pandemic when this novel drug has been authorized for the management of critical COVID-19 infections.
Acknowledgments
The authors would like to acknowledge the Department of Radiology and Department of Pathology at Saint Francis Presence Hospital for providing valuable input to this case presentation.
Financial Disclosure
The authors confirm that there is no funding to declare regarding the publication of this case report.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Informed Consent
Informed written consent was obtained from the patient to write and publish their case as a case report with all accompanying clinical and radiological images. No ethical clearance is deemed required for case report writing as per our local Research Board.
Authors Contributions
ES and AA contributed to the conceptualizing and writing the first manuscript. MA and AB have performed the critical review and editing of the final draft. All authors were involved in the clinical management of the reported patients. All authors agreed to the final draft submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Gout T, Ostor AJ, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol. 2011;30(11):1471-1474.
doi pubmed - Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68(11):2612-2617.
doi pubmed - Strangfeld A, Richter A, Siegmund B, Herzer P, Rockwitz K, Demary W, Aringer M, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis. 2017;76(3):504-510.
doi pubmed - Craig E, Cappelli LC. Gastrointestinal and hepatic disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2018;44(1):89-111.
doi pubmed - Curtis JR, Xie F, Chen L, Spettell C, McMahan RM, Fernandes J, Delzell E. The incidence of gastrointestinal perforations among rheumatoid arthritis patients. Arthritis Rheum. 2011;63(2):346-351.
doi pubmed - Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13(5):R141.
doi pubmed - Jagpal A, Curtis JR. Gastrointestinal perforations with biologics in patients with rheumatoid arthritis: implications for clinicians. Drug Saf. 2018;41(6):545-553.
doi pubmed - Group RC. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645.
doi - Bulte JP, Postma N, Beukema M, Inberg B, Stegeman AG, van der Hoeven H. COVID 19 and the risk of gastro-intestinal perforation: A case series and literature review. J Crit Care. 2022;67:100-103.
doi pubmed - Hofmaenner DA, Wendel Garcia PD, Ganter CC, Brugger SD, Buehler PK, David S. What every intensivist should know about Tocilizumab. Crit Care. 2021;25(1):262.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


