| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 4, April 2022, pages 192-195
Amphetamine Use Revealing Hypertrophic Cardiomyopathy in a Young Patient
Vulnet Misimia, g, Muhemin Mohammedb, g, Konstantinos Stamouc, Andreas Mitsisd, Panagiotis Sakellaropoulose, Despoina Tounissidouf, Stefanos G. Sakellaropoulosb, h
aDepartment of Internal Medicine, Stadtspital Waid und Triemli, Zurich, Switzerland
bSwiss Cardiovascular Centre, Bern University Hospital, Bern, Switzerland
cSchool of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
dCardiology Department, Nicosia General Hospital, Nicosia, Cyprus
eDepartment of Biopathology and Medical Biochemistry, Kalamata General Hospital, Kalamata, Greece
fDepartment of Pediatrics, Kalamata General Hospital, Kalamata, Greece
gThese authors have equally contributed to this article.
hCorresponding Author: Stefanos G. Sakellaropoulos, Swiss Cardiovascular Centre, Bern University Hospital, Bern, Switzerland,
Manuscript submitted February 12, 2022, accepted March 8, 2022, published online March 25, 2022
Short title: HCM in Patient With Chronic Use of Amphetamine
doi: https://doi.org/10.14740/jmc3915
| Abstract | ▴Top |
Adrenergic overstimulation in long term can lead to a hyperdynamic myocardium and give rise to hypertrophy and ultimately to heart failure. Amphetamine use is a common cause of neurohormonal activation, which gives rise to such adverse cardiovascular events. However, hypertrophy of myocardium in young patients, even due to apparently obvious causes, should always be considered as a red flag and a further diagnostic downstream should take place, in order to exclude genetic causes. We present a case of a young man with chronic use of amphetamine and an incidental finding of hypertrophic cardiomyopathy.
Keywords: Myopericarditis; Hypertrophic cardiomyopathy; Sudden cardiac death; Cardiac MRI
| Introduction | ▴Top |
Hypertrophic cardiomyopathy (HCM) is one of the most common genetically inherited diseases that affects the myocardium. The disease is caused by the mutation of genes that encode proteins for the cardiac sarcomere. It is the most frequent cause of sudden death of young people and trained athletes. For the purpose of risk and therapy stratification, the performance of diagnostic methods is required, in particular heart catheterization, transthoracic and transesophageal echocardiography, magnetic resonance imaging (MRI), genetic counselling and tissue biopsy. The appliance of these methods needs to be personalized depending on the patient’s phenotype and genotype.
In this paper, we present the case of a 30-year-old patient whose amphetamine “speed” intoxication led to myopericarditis. Amphetamine-induced myopericarditis is a rare cardiac complication. There are only few documented cases in literature. Other adverse effects reported in connection with the use of amphetamine-type stimulants (ATS) have included arrhythmia and cardiomyopathy (dilatative and takotsubo cardiomyopathy). Our diagnostic downstream analysis has shed light on an unexpected finding of HCM in cardiac imaging. Although age is an anti-risk factor in patients with HCM, careful evaluation and risk stratification would be essential to prevent sudden cardiac deaths. It would also be important to raise public awareness of this issue because cardiac complications may lead to morbidity and mortality of patients who have consumed ATS.
| Case Report | ▴Top |
Investigations
A previously healthy 30-year-old Caucasian man was admitted to the emergency department with a 6-h history of sudden onset crushing chest pain accompanied by radiation to the scapula and thoracic pain exacerbated by inspiration. The patient reported that on the preceding day he took amphetamine (speed). The patient consumed amphetamine more frequently in the weeks before the hospital admission, about twice per week. However, the patient could not precisely define the exact period and dosage of his amphetamine usage. Nevertheless, the patient’s urinary tests confirmed the use of amphetamine.
In the emergency room, the physical examinations did not lead to unusual findings. The patient’s blood pressure was 133/75 mm Hg, respiratory rate 27/min, peripheral oxygen saturation 97% and axillary temperature 37.2 °C. Cardiac and pulmonary auscultation showed no abnormality. The patient did not report about any previous cold symptoms, viral infection or any other kind of infections. The electrocardiogram (ECG) demonstrated a sinus rhythm with T-wave inversion in V2 - V5 and ST-segment depression in II, III and aVF. There were no previous ECG recordings available to exclude old alterations. Upon arrival, the laboratory investigation demonstrated elevated levels of high-sensitivity troponin T (hsTnT) 1,555 ng/L, creatine kinase-MB (CK-MB) 80.4 µg/L, C-reactive protein (CRP) 122 mg/L. Concerning the patient’s family history, there were no maternal or paternal predispositions for cardiovascular diseases, and no history of syncope or sudden cardiac deaths.
Diagnosis
The transthoracic echocardiography found an asymmetric hypertrophy of the septum wall with normal ejection fraction and normal valve structures. There were no signs of the right ventricular enlargement or wall motion abnormalities. With regard to the age and reported symptoms, as well as ECG changes, we performed a coronary computed tomography (CCT) to exclude any coronary artery disease or coronary anomalies. As the patient had normal coronary anatomy, calcification and stenosis of coronaries were excluded.
Due to the high suspicion of a myopericarditis and the unexplained septum hypertrophy, we performed cardiac MRI (CMRI). The CMRI images showed both normal left and right systolic functions, and normal dimensions. There was no evidence of wall motion abnormalities for either ventricle. However, the CMRI images revealed an asymmetric septal hypertrophy over 15 mm with intramural late gadolinium enhancement (LGE), both basal and mid-ventricular, as well as in the right ventricular insertion points. These findings strongly suggested a diagnosis of HCM (Fig. 1a-c). Due to artifact overlay, neither the T1 and T2 mapping, nor the T2-weighted sequences were assessable.
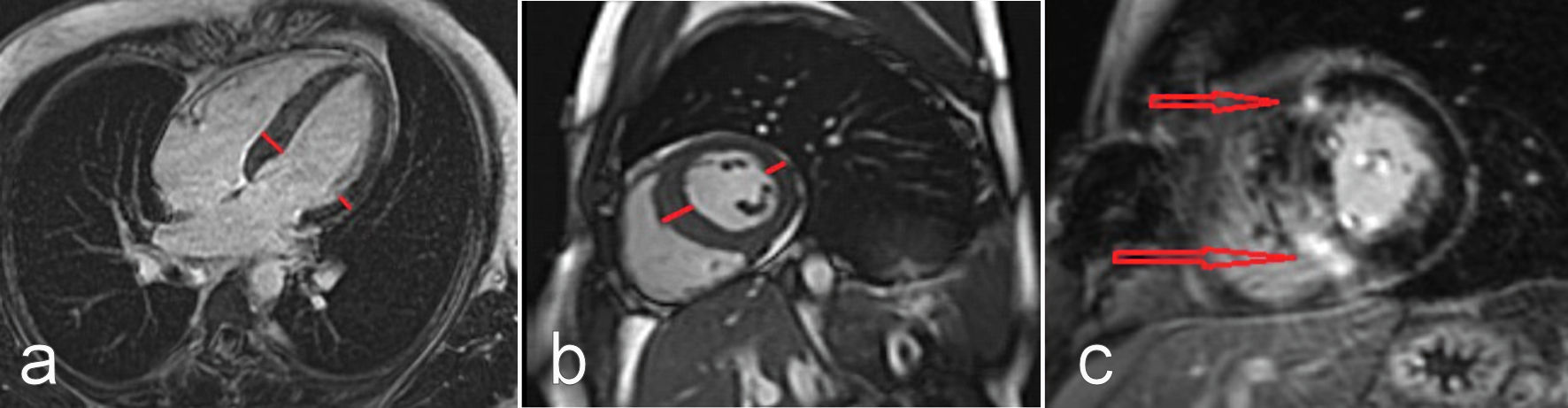 Click for large image | Figure 1. CMRI long axis four-chamber view (a), and short axis view at the level of papillary muscles (b): asymmetric septal hypertrophy (as shown by short lines) is appreciated. Gadolinium-enhanced CMRI short axis view at the level of papillary muscles (c): late gadolinium enhancement on the right side and ventricular insertion points can be appreciated (as shown by arrows), typically indicating hypertrophic cardiomyopathy. CMRI: cardiac magnetic resonance imaging. |
Overall, the symptoms, the clinical pictures, and the laboratory tests, along with the patient’s drug-history, underpinned the probability of an amphetamine-induced myopericarditis.
Treatment
Following the diagnosis of acute myopericarditis, the patient was given beta-blocker and non-steroidal anti-inflammatory drug. As CMRI did not detect any effusion, a treatment escalation with colchicine was not required. Over the course of the next 48 h, the patient’s chest pain resolved, CK levels normalized, and hsTnT decreased. Eventually, the patient was discharged with the diagnosis of an amphetamine-induced myopericarditis with HCM.
Considering therapeutic approaches of HCM, medical treatment includes beta-blocker; verapamil in case of side effect due to beta-blocker or noncardiac contraindication of beta-blocker; and additional disopyramide in case of persistent left ventricular outflow tract (LVOT) gradient, persistent symptoms, and absence of contraindication to disopyramide. In patients with refractory symptoms and an already existing pacemaker, dual-chamber (DDD) pacemaker upgrade with short atrioventricular delay is an option. Furthermore, patients may need a more invasive management and may have to undergo myectomy or alcohol septal ablation (ASA) [1].
In patients with systolic gradients 50 mm Hg who are not possible to control their symptoms with pharmacotherapy or who have side effects, septal myectomy is recommended. At this stage a very careful examination of mitral valve (MV) apparatus is essential. Preoperative echocardiography and MRI can discover the mitral abnormalities in patients with HCM. This is very important as it can directly lead to a judgment for surgical septal myectomy rather than ASA which only addresses the septal thickening. In case of distinctive and pronounced MV abnormalities, ASA alone perhaps may be not sufficient and surgical MV repair in association with septal myectomy is probably the preferred approach [1].
Finally, taking however into account that ASA has exceptionally good results, the final judgment call belongs to the patient after providing him precise information and clarification of both septal ablation and myectomy, ideally in expert HCM centers.
Follow-up and outcomes
Shortly after discharge, we arranged for the patient a first follow-up visit for further examinations as part of a dedicated special consultation service for HCM patients. The purpose of this visit was to draw a detailed family history and pedigree analysis with the closer family involved. In such follow-up visits, we perform echocardiography, cardiopulmonary exercise testing (CPET) and Holter test. In this particular case, we did not find any family history of heart disease, such as HCM or sudden cardiac death. In addition, we suggested genetic testing for accurate diagnosis and therapeutic and prognostic consequences. In our clinic, we perform HCM genetic test broad panel. However, the patient and the family concerned refused genetic testing. And so, a yearly follow-up is arranged, including echocardiography, CPET und Holter test. The main purpose would be to detect a possible risk factor for sudden cardiac death and establish an indication for an implantation of an implantable cardioverter defibrillator for primary prophylaxis.
| Discussion | ▴Top |
ATS, such as amphetamine, methamphetamine or 3, 4-methylenedioxymethamphetamine (MDMA) poses significant problems for health services worldwide. They are the second most commonly used illegal drugs after cannabis in the European Union. In 2020, lifetime prevalence of ATS use was estimated at 13.6 million (4.1%) for MDMA and 12.3 million (3.7%) for amphetamines among 15 - 64 years old group. Furthermore, in the case of young adults (15 - 34 years old age group), this number was estimated to reach 2.3 million (1.9%) for MDMA and 1.4 million (1.2%) for amphetamines [2]. From a societal perspective, chronic ATS use may lead to significant social harm, even criminal offences. In terms of medical impairments, it may cause malnutrition, mental illnesses, and cardiovascular diseases.
The relevant literatures have highlighted the increased occurrence of amphetamine-associated cardiomyopathy [3-5]. Amphetamine-induced vasospasm and ischemia, direct toxicity by lysis and necrosis of the myocytes, as well as deleterious effects of excess catecholamines on cardiomyocytes are the most important causes of acute and chronic cardiomyopathy [6-8]. In terms of its effects, amphetamine exerts its sympathomimetic effects indirectly by causing increased release of dopamine, norepinephrine, epinephrine, and serotonin [7, 9]. This generates a pleasurable emotional response for the user, which is linked to improved mood and increased energy.
Amphetamine has been linked to various cardiac pathologies. High blood pressure and increased heart rate appear to increase by increasing amphetamine doses due to its adrenergic stimulation [7, 10]. Other possible pathologies include arrhythmias, vasospasm, accelerated atherosclerosis, acute coronary syndrome, circulatory collapse, as well as cardiomyopathy. Rajs et al has described pathologic findings, including cardiac chamber enlargement, left ventricular hypertrophy, hemorrhage, fibrosis, and contraction band necrosis in 14 subjects who had used centrally acting amines such as amphetamine [11].
From a pathophysiological perspective, amphetamine use in patients with hypertrophic obstructive cardiomyopathy could lead to devastating outcomes. A hyperdynamic myocardium may cause increased left ventricular outflow obstruction, as well as overstimulation of the arrhythmogenic myocardial disarray, which may ultimately cause sudden cardiac death. As described above, chronic use of amphetamine could potentially cause a left ventricular hypertrophy. Such a mechanism could be the reason for the left ventricular hypertrophy in our case. However, in this particular case, the hypertrophy is primarily located in the septum. Localized septal hypertrophy with presence of LGE is the most frequent pattern in HCM [11, 12]. For this reason, adrenergic overstimulation is the primary target of the medical strategy in patients with HCM. In case of adrenergic overstimulation, a global concentric left ventricular hypertrophy should be expected [13] (Fig. 1a-c).
Conclusions
The use of ATS has been on the rise in the past few decades. Several studies have explored the potential effects of ATS on cardiac toxicity leading to acute myocardial injury or severe form of cardiomyopathy. Amphetamine-associated cardiomyopathy is a potentially fatal complication caused by the use of amphetamine. As described above, left ventricular hypertrophy can be the result of adrenergic overstimulation. However, in the case of young patients, left ventricular hypertrophy should always trigger the suspicion of other causes, such as in our case, HCM. Asymmetric hypertrophy is highly pathognomonic, so in this regard, further diagnostic work should take place. CMRI has emerged over the last decade as one of the most important cardiac modalities in cardiology and belongs to the diagnostic armamentarium, especially in case of suspected HCM. Furthermore, CMRI is essential to detect abnormalities in MV (elongated mitral leaflets, displacement of papillary muscles, and systolic anterior motion), which may be the primary pathognomonic elements, even in the absence of hypertrophy [1]. CMRI can also detect morphological abnormalities in case of HCM, which are highly pathognomonic for increased risk for sudden cardiac death, such as left ventricular aneurysms, left ventricular crypts, percent of LGE [14]. Finally, we strongly advise the performance of CPET. Pathological parameters, especially decreased oxygen consumption and lately reported the presence of exercise oscillatory ventilation are considered as markers of disease severity and reflect an increased risk for sudden cardiac death [15]. This case highlights that chronic drug users would need to have more frequent cardiological checks. On a broader level, these medical findings could help to reach a more holistic and factually reasoned social consensus about drug prevention programs related to the use of ATS.
Acknowledgments
We thank our colleagues from Inselspital for providing the images of the manuscript.
Financial Disclosure
None to declare.
Conflict of Interest
Authors have no conflict of interest to declare.
Informed Consent
Informed consent was obtained from the patient to publish this report.
Author Contributions
Vulnet Misimi, Konstantinos Stamou, Andreas Mitsis, Panagiotis Sakellaropoulos and Despoina Tounissidou have contributed to this case report and discussion. Muhemin Mohammed has especially contributed to this article concerning imaging modalities, cardiac MRI. Stefanos G Sakellaropoulos and Muhemin Mohammed have both contributed to the article design, providing clinical expertise to revise critically.
Data Availability
The data are available from the corresponding author upon reasonable request.
Abbreviations
ATS: amphetamine-type stimulants; CMRI: cardiac magnetic resonance imaging; CT: computed tomography; HCM: hypertrophic cardiomyopathy; LGE: late gadolinium enhancement
| References | ▴Top |
- Sakellaropoulos S, Svab S, Mohammed M, Dimitra L, Mitsis A. The role of mitral valve in hypertrophic obstructive cardiomyopathy: an updated review. Curr Probl Cardiol. 2021;46(3):100641.
doi pubmed - European drug report 2020. Monitoring Centre for Drugs and Drug Addiction. 2020. https://www.emcdda.europa.eu/edr2020_en.
- Schurer S, Klingel K, Sandri M, et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine - associated cardiomyopathy. JACC Heart Fail. 2017;5:435-445.
doi pubmed - Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41(7):981-986.
doi pubmed - Reddy PKV, Ng TMH, Oh EE, Moady G, Elkayam U. Clinical Characteristics and management of methamphetamine-associated cardiomyopathy: State-of-the-Art Review. J Am Heart Assoc. 2020;9(11):e016704.
doi pubmed - Jafari Giv M. Exposure to Amphetamines Leads to Development of Amphetamine Type Stimulants Associated Cardiomyopathy (ATSAC). Cardiovasc Toxicol. 2017;17(1):13-24.
doi pubmed - Kevil CG, Goeders NE, Woolard MD, Bhuiyan MS, Dominic P, Kolluru GK, Arnold CL, et al. Methamphetamine use and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2019;39(9):1739-1746.
doi pubmed - Ansari A, Maron BJ, Berntson DG. Drug-induced toxic myocarditis. Tex Heart Inst J. 2003;30(1):76-79.
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681-698.
doi pubmed - Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33(8):1847-1855.
doi pubmed - Rajs J, Falconer B. Cardiac lesions in intravenous drug addicts. Forensic Sci Int. 1979;13(3):193-209.
doi - Mihl C, Dassen WR, Kuipers H. Cardiac remodelling: concentric versus eccentric hypertrophy in strength and endurance athletes. Neth Heart J. 2008;16(4):129-133.
doi pubmed - Syed IS, Ommen SR, Breen JF, Tajik AJ. Hypertrophic cardiomyopathy: identification of morphological subtypes by echocardiography and cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2008;1(3):377-379.
doi pubmed - Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Dearani JA, et al. Management of hypertrophic cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79(4):390-414.
doi pubmed - Sakellaropoulos SG, Baggish AL, Fifer MA, Lewis GD. Exercise oscillatory ventilation in hypertrophic cardiomyopathy. Curr Probl Cardiol. 2021.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


