| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 4, April 2022, pages 188-191
Pneumatosis Cystoides Intestinalis: A Benign Cause of Pneumoperitoneum
Priyanthi Widana Pathiranaa, b, c, Adrian Fernandeza
aSouth East Regional Hospital, Bega, NSW, Australia
bDepartment of General Surgery, Prince of Wales Hospital, Randwick, NSW, Australia
cCorresponding Author: Priyanthi Widana Pathirana, Department of General Surgery, Prince of Wales Hospital, Randwick, NSW 2031, Australia
Manuscript submitted February 26, 2022, accepted March 21, 2022, published online March 25, 2022
Short title: PCI as a Benign Cause of Pneumoperitoneum
doi: https://doi.org/10.14740/jmc3919
| Abstract | ▴Top |
Pneumatosis cystoides intestinalis (PCI) is an uncommon condition where cyst-like locules of gas are present in the submucosa or subserosa of the gastrointestinal tract. The majority of cases are benign and are associated with drugs such as chemotherapy agents or conditions including chronic obstructive airways disease. We present the case of PCI resulting in pneumoperitoneum in a 72-year-old male patient on chemotherapy for esophageal adenocarcinoma. While he was noted to have an extensive pneumoperitoneum and mesenteric emphysema on imaging, he remained clinically stable with a benign abdominal examination and was managed conservatively with intravenous antibiotics and fluids. This case highlights the importance of benign PCI as a differential to bowel ischemia in patients with pneumatosis intestinalis and pneumoperitoneum, particularly given the possibility of avoiding operative management and the resultant morbidity.
Keywords: Pneumatosis cystoides intestinalis; Chemotherapy; Pneumoperitoneum; Pneumatosis intestinalis
| Introduction | ▴Top |
Pneumatosis cystoides intestinalis (PCI) is an uncommon condition where cyst-like locules of gas are present in the submucosa or subserosa of the gastrointestinal tract. The majority of cases are benign and are associated with drugs such as chemotherapy agents or conditions including chronic obstructive airways disease [1, 2]. As in fulminant cases such as those secondary to bowel ischemia, benign PCI can result in pneumoperitoneum, mesenteric or retroperitoneal free gas and is an important differential in clinically well patients given the possibility of non-operative management [2]. We present a case of PCI resulting in pneumoperitoneum in a 72-year-old male patient on chemotherapy for esophageal adenocarcinoma. Despite his radiological findings, he remained clinically well with normal hemodynamics and a benign abdominal examination which prompted a conservative approach to management that was successful.
| Case Report | ▴Top |
Investigations
A 72-year-old male patient currently undergoing neoadjuvant chemo-radiotherapy (CROSS protocol) for distal esophageal adenocarcinoma was referred to hospital after being incidentally noted to have a pneumoperitoneum on an outpatient computed tomography (CT) of chest, abdomen and pelvis. He had completed his final round of chemotherapy 1 week prior and had undergone the CT for staging prior to a multidisciplinary team meeting at which stage he was to be considered for an esphagectomy. Relevant past medical history included a previous smoking history with emphysematous changes on CT; however, he was not on any treatment and had a reasonable exercise tolerance being able to walk up two flights of stairs. He had also been treated for lung adenocarcinoma with a left upper lobectomy a decade prior. He was not on any regular medications. Confounding his presentation was his history of having a jejunostomy inserted 3 months previously for feeding due to dysphagia from his partially obstructing distal esophageal malignancy.
On presentation, he reported increasing shoulder tip pain over the past 48 h and generalized lethargy which was not dissimilar to following his previous rounds of chemotherapy. He denied worsening abdominal pain and fevers, however did report the development of non-blood containing diarrhea. He was afebrile and hemodynamically stable with a heart rate of 94 bpm, blood pressure of 129/86 mm Hg and oxygen saturation of 96% on room air. He was comfortable and mobilizing independently around the emergency department. On examination, he was clinically dehydrated and was noted to have a mildly distended abdomen which was tender diffusely across the upper abdomen without peritonism.
Diagnosis
He was found to be leukopenic in the setting of being 1 week post treatment with carboplatin and paclitaxel with a white cell count of 1.5 × 109/L. He had a normal acid-base balance on venous blood gas, no electrolyte abnormalities and slightly elevated C-reactive protein of 16 mg/L. His intravenous contrast CT showed a pneumoperitoneum predominantly in the right subhepatic space with extensive mesenteric gas and intramural gas locules predominantly within the hepatic flexure (Fig. 1). There was no evidence of free fluid, bowel wall thickening or hypoenhancement to suggest ischemia.
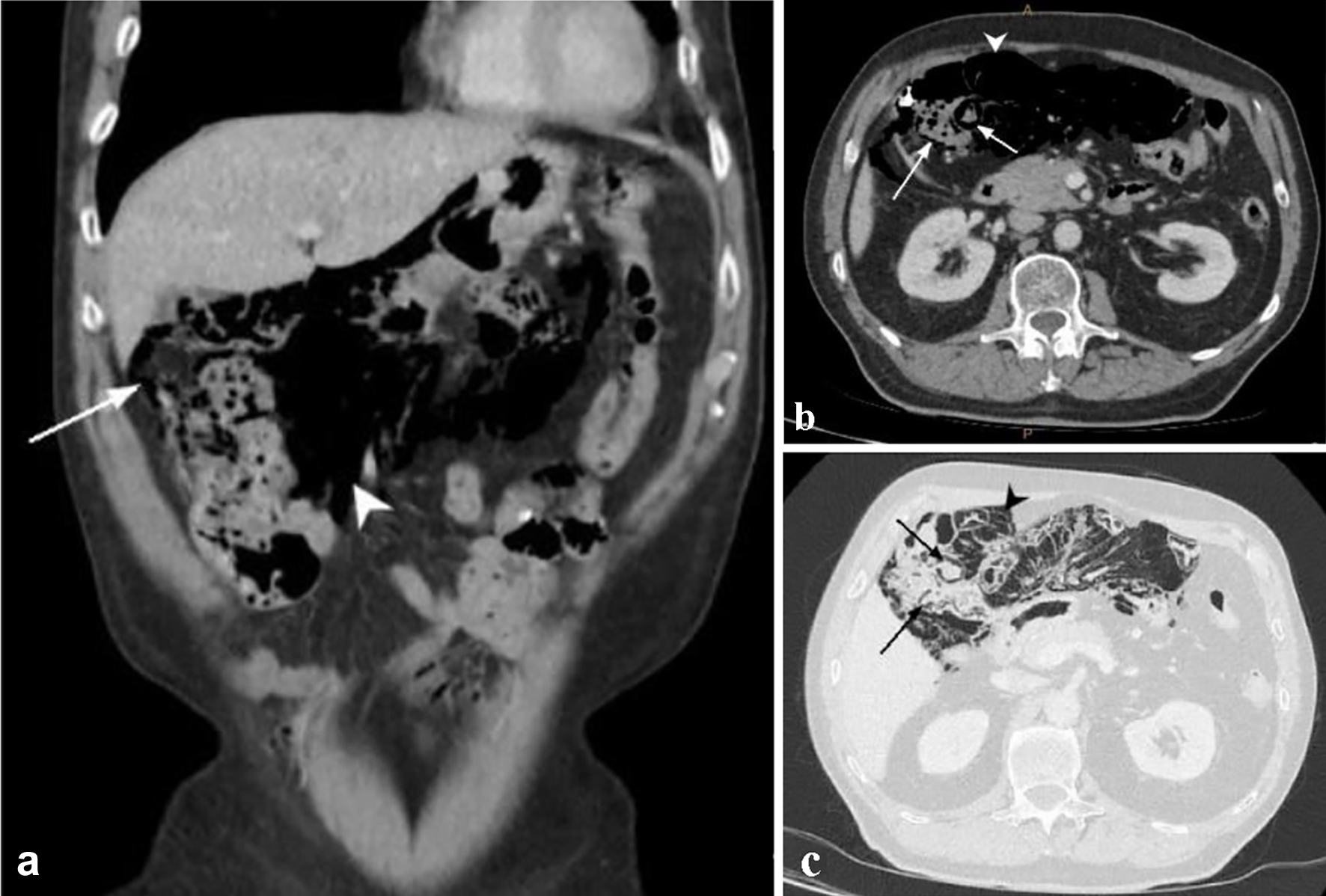 Click for large image | Figure 1. Contrast-enhanced CT of abdomen and pelvis: (a) coronal view in soft tissue window, (b) axial view in soft tissue window, (c) axial view in lung window, showing intramural locules of gas within the right sided colon (arrow) and extensive gas within the mesocolon and mesentery (arrowhead) and pneumoperitoneum outlining the liver margin. CT: computed tomography. |
Treatment
While his immunosuppression was potentially masking clinical and biochemical signs of bowel ischemia, his examination and history were reassuring and the decision was made to proceed with non-operative treatment. He was kept fasting with his jejunostomy feeds suspended, commenced on nasal prong oxygen, intravenous fluids and intravenous piperacillin/tazobactam given his neutropenia. Given he remained clinically well overnight with a soft abdomen on examination the next morning, he was recommenced on his jejunostomy feeds. Over the next 72 h his abdominal tenderness resolved and his inflammatory markers remained stable and on day 3 his antibiotics were de-escalated to amoxicillin/clavulanic acid administered via his jejunostomy. His diarrhea however was ongoing and after his stool bacterial and viral polymerase chain reaction (PCR) and Clostridium difficile toxin returned as negative, he was commenced on loperamide with good effect.
Follow-up and outcomes
He was discharged home on day 6 of admission on a further 5 days of antibiotics with follow-up arranged with his upper gastrointestinal surgeon and medical oncologist. He underwent a repeat CT of abdomen and pelvis 2 months post admission which noted resolution of PCI, pneumoperitoneum and mesenteric gas.
| Discussion | ▴Top |
PCI refers to the radiological findings of cyst-like intramural gas locules within the intestinal tract, commonly within the small intestine and colon but can occur from esophagus to rectum [1]. Approximately 15% are idiopathic whilst secondary PCI is associated with a variety of etiologies as outlined in Table 1, including gastrointestinal causes such as enterocolitis, ischemia and gastrointestinal surgery. Pharmacological causes include anti-neoplastic agents as in this case, steroids or immunosuppressants and alpha-glucosidase inhibitors. Non-gastrointestinal causes include obstructive airways disease, hematological malignancies, acquired immune deficiency syndrome and autoimmune conditions perhaps due to the use of glucocorticoids [2, 3].
 Click to view | Table 1. Causes of Secondary PCI |
While the exact pathophysiology remains unclear, it is believed to relate to one or more of the following: mucosal injury, gas producing bacteria and increased intestinal pressure such as from intestinal obstruction or airway obstruction [1, 2]. In the setting of chemotherapy, the development of mucositis with reduced mucosal integrity and altered intestinal flora coupled with underling immunosuppression may play a role in the development of PCI [3-5]. Additionally, the use of glucocorticoids in chemotherapy may result in atrophy of the intestinal mucosa, hence predisposing to greater susceptibility of the mucosa to increased intestinal pressure resulting in submucosal gas [6]. Interestingly, the described patient had a background of recent chemotherapy and obstructive airways disease, both of which are associated with benign PCI. His jejunostomy was believed less likely to be contributing to his presentation given it had been 3 months since insertion and the mesenteric gas was predominantly around the right upper quadrant of the abdomen, including the omentum and mesocolon.
The clinical findings of PCI are often non-specific if present at all. A systematic review by Wu et al of PCI within the Chinese population found abdominal pain, distension and diarrhea to be the most common symptoms [7]. Diagnosis has largely been reliant on radiological investigations; however, endoscopy is playing an increasing role allowing for the direct visualization of submucosal cysts which present as polypoid structures. While these cysts can be mistaken for polyps or inflammatory bowel disease, they are readily apparent on endoscopic ultrasound [2]. X-ray may be sufficient to visualize PCI given the radio-opaque nature of the intramural cysts, particularly when outlined in the setting of a barium enema, and can identify complications including pneumoperitoneum. CT identifies the intramural gas consistent with PCI, particularly on a lung window and identifies signs of spontaneous perforation including pneumoperitoneum, pneumoretroperitoneum or mesenteric gas. Where CT is of particular utility compared to alternative imaging modalities or endoscopy is in the identification of features suggestive of fulminant PCI [7-9].
Management of PCI relies on distinguishing between benign and fulminant causes with intestinal ischemia being the primary aetiology of concern, particularly in the setting of peritonitis, hemodynamic instability or leukocytosis. Concerning features on CT more consistent with fulminant PCI include free fluid, reduced bowel wall enhancement, bowel wall edema and portal venous gas all of which were absent in the presented case and strongly suggest the need for operative intervention [8, 9].
Asymptomatic patients require no intervention, or may be managed conservatively with supplemental oxygen including hyperbaric oxygen; to increase the partial pressure of oxygen and reduce the partial pressure of other gases in the circulatory system facilitating the diffusion of gas from the cysts into the blood stream; antibiotics to reduce the intestinal gas-producing bacterial load and fluid replacement in the setting of diarrhea [1, 2, 7]. Additionally, endoscopy is increasingly being used therapeutically by facilitating aspiration or excision of cysts [2, 10]. While endoscopic intervention may not be feasible in the often-widespread nature of PCI, it has a role in managing possible sequelae including intestinal obstruction from large cysts or preventing a volvulus from cysts acting as a fulcrum [10]. The incidence of complication such as perforation or obstruction is reported as 16.3% by Wu et al and as in the presented case, may still be managed conservatively [7]. Surgery should be reserved for patients showing signs of peritonism or sepsis or for cases of persistent bowel obstruction either not amenable to, or recurrent after endoscopic intervention [7].
Ultimately the rate of recurrence is high particularly in primary PCI, and the main stay of treatment is to relieve the underlying cause in secondary PCI such as cessation of chemotherapy as in this instance [1]. In conclusion, by presenting this case we aim to inform clinicians of this uncommon and often benign cause of pneumoperitoneum and highlight the potential for non-operative management in order to reduce the morbidity associated with unnecessary surgery.
Learning points
PCI is an uncommon radiological and endoscopic finding and often follows a benign cause. While bowel ischemia is an important consideration in the setting of a pneumoperitoneum in a patient undergoing chemotherapy, PCI remains an important differential, particularly in the setting of a clinically well patient. Correctly identifying PCI may allow patients to be managed non-operatively thus avoiding the morbidity involved with surgical intervention.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was gained prior to completion of the case report.
Author Contributions
PWP contributed to writing, editing and conception of the case report. AF contributed to the conception and editing of the case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PCI: pneumatosis cystoides intestinalis; CT: computed tomography
| References | ▴Top |
- Galandiuk S, Rice J, Deveaux P, Farmer R. Pneumatosis cystoides intestinalis. In: Fazio VW, Church JM, Delaney CP, Kiran RP, editors. Current therapy in colon and rectal surgery. 3rd ed. Philadelphia, PA: Elsevier; 2017. p. 345-348.
- Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176.
doi pubmed - Groninger E, Hulscher JB, Timmer B, Tamminga RY, Broens PM. Free air intraperitoneally during chemotherapy for acute lymphoblastic leukemia: consider pneumatosis cystoides intestinalis. J Pediatr Hematol Oncol. 2010;32(2):141-143.
doi pubmed - Mimatsu K, Oida T, Kawasaki A, Kano H, Kuboi Y, Aramaki O, Amano S. Pneumatosis cystoides intestinalis after fluorouracil chemotherapy for rectal cancer. World J Gastroenterol. 2008;14(20):3273-3275.
doi pubmed - Lee YS, Han JJ, Kim SY, Maeng CH. Pneumatosis cystoides intestinalis associated with sunitinib and a literature review. BMC Cancer. 2017;17(1):732.
doi pubmed - Wang YJ, Wang YM, Zheng YM, Jiang HQ, Zhang J. Pneumatosis cystoides intestinalis: six case reports and a review of the literature. BMC Gastroenterol. 2018;18(1):100.
doi pubmed - Wu LL, Yang YS, Dou Y, Liu QS. A systematic analysis of pneumatosis cystoids intestinalis. World J Gastroenterol. 2013;19(30):4973-4978.
doi pubmed - Azzaroli F, Turco L, Ceroni L, Galloni SS, Buonfiglioli F, Calvanese C, Mazzella G. Pneumatosis cystoides intestinalis. World J Gastroenterol. 2011;17(44):4932-4936.
doi pubmed - Lee KS, Hwang S, Hurtado Rua SM, Janjigian YY, Gollub MJ. Distinguishing benign and life-threatening pneumatosis intestinalis in patients with cancer by CT imaging features. AJR Am J Roentgenol. 2013;200(5):1042-1047.
doi pubmed - Takahashi K, Fujiya M, Ueno N, Ando K, Kashima S, Moriichi K, Okumura T. Endoscopic fine-needle aspiration is useful for the treatment of pneumatosis cystoides intestinalis with intussusception. Am J Gastroenterol. 2019;114(1):13.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


