| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 6, June 2022, pages 307-311
The Impact on COVID-19 by Intravenous Bevacizumab Used for Hereditary Hemorrhagic Telangiectasia: A Case Report
Ryan Patrick Fanninga, b, Sara Stroutb, Nicholas R. Rowanb, Clifford R. Weissb, Panagis Galiatsatosa, b, c
aDivision of Pulmonary and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA
bThe Hereditary Hemorrhagic Telangiectasia Center for Clinical Excellence, Johns Hopkins School of Medicine, Baltimore, MD, USA
cCorresponding Author: Panagis Galiatsatos, Division of Pulmonary and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21224, USA
Manuscript submitted April 13, 2022, accepted May 12, 2022, published online June 2, 2022
Short title: Intravenous Bevacizumab Used for HHT
doi: https://doi.org/10.14740/jmc3948
| Abstract | ▴Top |
Coronavirus disease 2019 (COVID-19) continues as an infectious pandemic. With emphasis on mitigating its impact globally, strategies have been emphasized on prevention to treatment in severe cases. As for pharmacotherapies, many have been researched, with a few being recommended for patients with COVID-19 depending upon their severity. Bevacizumab, a recombinant monoclonal antibody often used for oncological disease and rare genetic disorders, has gained attention in combatting COVID-19 due to the pharmacotherapy’s ability to inhibit vascular endothelial growth factor A (VEGF-A). VEGF has been identified as significantly upregulated in the lungs of persons who have died of COVID-19, raising interest for VEGF to be a potential target for patients with COVID-19. We present a case of a patient who was admitted due to complications of a rare genetic disorder, called hereditary hemorrhagic telangiectasia (HHT), warranting intravenous bevacizumab, who subsequently was diagnosed with COVID-19 pneumonia. We discuss the patient’s outcome and contribute to the growing potential of bevacizumab in the treatment of COVID-19.
Keywords: COVID-19; Bevacizumab; Hereditary hemorrhagic telangiectasia
| Introduction | ▴Top |
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the virus that causes coronavirus disease 2019 (COVID-19), and the resulting global public health crisis [1]. In almost all patients with severe COVID-19, inflammatory pulmonary edema results in dyspnea and hypoxemia, warranting immediate medical attention [2, 3]. Such a consequence is due to a disruption of the pulmonary endothelial cells, which function as a basic barrier between blood and interstitium [4]. The targeting of these cells, which when disrupted result in primary pulmonary pathologies, has been identified as a key pathway for the adverse effects of SARS-CoV-2 infection and resulting COVID-19 pathogenesis by altering the endothelial barrier’s integrity [5]. Therefore, potential pharmacotherapies that offer the ability to maintain and preserve the pulmonary endothelial barrier may have a potential role in the management of COVID-19.
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant, multi-systemic disorder with a prevalence of 12.1 per 100,000 persons, characterized by arteriovenous malformations (AVM) with clinical consequences, from hemorrhaging to infections [6]. The AVMs of HHT form due to a lack of capillaries, resulting in a direct connection between the veins and arteries [7]. Small AVMs are referred to as telangiectasias, with an abundance of them often found in the nasal passage and contributing to HHT’s most common symptom, epistaxis [8]. In advanced and refractory HHT, patients may warrant antiangiogenic pharmacotherapies in an effort to resolve AVMs and their resulting negative clinical outcomes (e.g., iron deficiency anemia) [7, 9]. One of the often used antiangiogenic agents, bevacizumab, is a monoclonal antibody that targets vascular endothelial growth factor (VEGF), a well-established pro-angiogenic protein that plays a significant role in endothelial barrier integrity and resulting vascular malformations of patients with HHT [10]. In that regard, bevacizumab’s ability to preserve endothelial integrity, as evidenced by its success in patients with HHT [11], may warrant attention in treating patients with COVID-19, where VEGF has been identified as a factor involved in the pathology of COVID-19 [4, 12]. SARS-CoV-2’s infection results in a significant release of pro-inflammatory cytokines and pro-angiogenic factors, such as VEGF [13]. The overexpression of VEGF in COVID-19 is thought to contribute to the resulting endothelial barrier disruption of the lungs, resulting in pulmonary edema, hypoxemia, and vascular remodeling [13].
We present a case of a patient with HHT who was admitted for severe epistaxis and found to have COVID-19 with significant pulmonary involvement. In this case presentation, we discuss the patient’s outcome after receiving intravenous bevacizumab, received for his HHT-related acute pathology, on his outcome from COVID-19.
| Case Report | ▴Top |
Investigations
Our patient is a 52-year-old male with a history of HHT. The patient’s main HHT-related morbidity is severe epistaxis that has resulted in iron deficient anemia, managed over the last 2 years with infusions of iron and transfusions of packed red blood cells (pRBC). Recently, the patient’s epistaxis was controlled with intravenous bevacizumab (5 mg/kg), with the patient completing six treatments of the pharmacotherapy (each treatment coming at 2-week intervals) between September and November of 2021. During these months, the patient’s hemoglobin peaked to 7.7 g/dL without the need for iron infusions or pRBC transfusions (last given August 2021, when the patient’s hemoglobin was 6.7 g/dL).
However, in December of 2021 (6 weeks since his last bevacizumab treatment), the patient began to experience worsening large volume blood loss from his epistaxis, resulting in an admission to the hospital for control of his epistaxis and its sequelae. Upon arrival to the hospital, the patient’s peak temperature was recorded at 38.0 °C (peak at 39.4 °C), heart rate at 118 beats per minute, blood pressure at 119/64 mm Hg, respiratory rate at 24 breaths per minute, and pulse oximeter reading 99% on room air. Given the surge of COVID-19 cases regionally, the patient was screened for SARS-CoV-2 by PCR, which resulted in a positive finding of the virus.
On initial laboratory evaluation, the patient’s hemoglobin was 3.8 g/dL, with an epistaxis severity score (HHT-ESS) of 9.49 (note a score greater than 7.00 is considered severe, with range 0 to 10). The patient’s white blood cell count and platelet count were both within normal range. However, in the white blood cell differential, there was an elevated neutrophil percentage at 77.5% (normal range 40-70%) and a reduced lymphocyte percentage at 10.8% (normal range 24-44%). Ferritin level was reported at 2 ng/mL (normal range 30 - 400 ng/mL), iron at 15 µg/dL (normal range 65 - 170 µg/dL), and iron saturation at 3% (normal range 20-55%). His complete metabolic panel, taking into account electrolytes, renal function markers, and hepatic markers, was unremarkable. Given the patient’s positive SARS-CoV-2 finding and reports of dyspnea, a workup for COVID-19 ensued to evaluate if it was contributing to the patient’s condition. The patient received a computed tomography (CT) of his chest with intravenous contrast, resulting in the finding of ground glass opacification of the right lower lobe (Fig. 1). Of note, the patient had received two doses of the BNT162b2 vaccine (mRNA-based vaccine), with his last dose in August 2021.
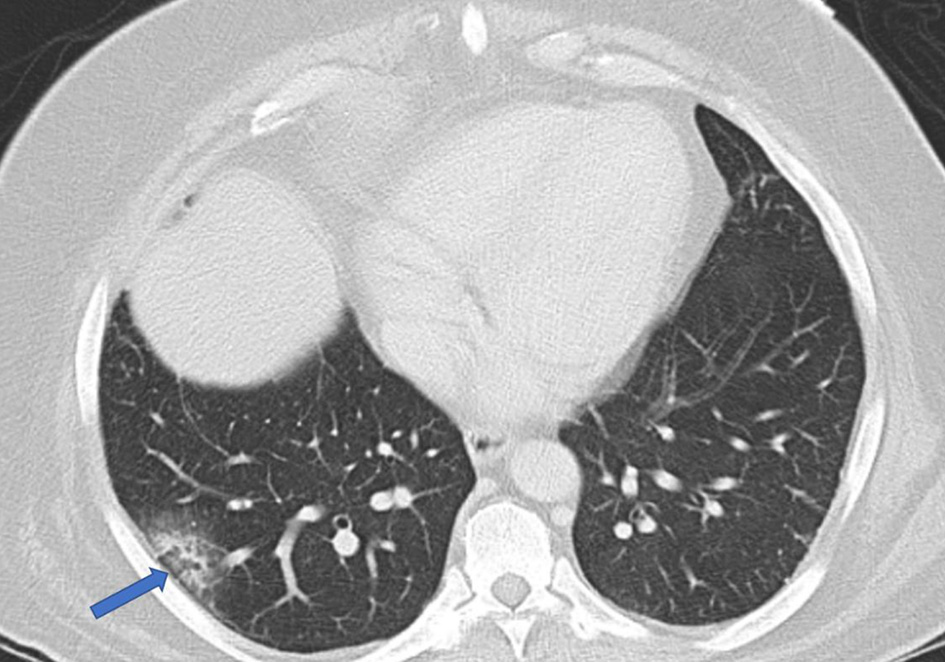 Click for large image | Figure 1. Computed tomography scan of the chest revealing ground glass opacification in the right lower lobe (arrow). |
Diagnosis
The patient was diagnosed with severe iron deficient anemia due to epistaxis from his HHT. In addition, he was diagnosed with moderate COVID-19 given his positive viral PCR, vital signs, and imaging findings.
Treatment
The patient was hospitalized for 5 days. In regards to his severe anemia from his epistaxis as a result of his HHT, he was treated with iron infusions and pRBC transfusions, receiving a total of 5 units of pRBC over the 5-day hospitalization. He also received intravenous bevacizumab, dosed at 5 mg/kg (standard dose for the treatment of epistaxis from HHT [11, 14]), on his second hospital day. At the time of discharge, his hemoglobin was at 8.1 g/dL. The rest of his blood work continued to be unremarkable and the patient continued to be on room air during the course of his hospitalization. For his moderate COVID-19, he was given remdesivir on hospital day 2, as the treatment was standard for hospitalized patients with moderate COVID-19 [15].
Follow-up and outcomes
The patient continues to be followed in clinic for his HHT, with his current hemoglobin at 9.4 g/dL. Further, he continues to receive intravenous bevacizumab every 2 weeks, which has been ongoing since his hospital discharge. Of note, he has no COVID-19-related sequelae.
| Discussion | ▴Top |
COVID-19 is associated with significant pulmonary dysfunction in its disease spectrum, ranging from infiltrates to pneumonia to acute respiratory distress syndrome (ARDS) [3]. Many pharmacotherapies have targeted non-viral host factors that become aberrant and pathogenic in the spectrum of COVID-19, such as dexamethasone (broad immunosuppressant target), tocilizumab (IL-6 target) [15]. In this context, VEGF, a factor that has been associated with COVID-19, and its likely disease progression from mild to moderate [13], seems to be a logical progression as a target candidate. In our case, we describe the use of bevacizumab in a patient warranting the medication due to HHT-related complications and the patient’s resulting outcome related to the patient’s subsequent moderate COVID-19 diagnosis.
Pang et al recently described the efficacy and tolerability of bevacizumab (dosed at 7.5 mg/kg) in patients with severe COVID-19 between February and April 2020 [16]. In this trial, 27 patients were enrolled and received one dose of bevacizumab and monitored over a 28-day period. In regards to efficacy of the intervention, the authors found 24 patients having improvement in their oxygen-support and no patient deaths were reported. Of note, the oxygen improvement in the patients became evident within 24 h of the bevacizumab treatment and none of the patients experienced a worsening in the oxygenation status who received bevacizumab. With safety, there were many adverse events that were reported, with the most common adverse event being abnormal hepatic laboratory data (30% of patients). In the context of this trial, our case report contributes uniquely to the growing evidence of bevacizumab’s potential in COVID-19 through the emphasis of three key factors: timing of the medication, dosing of the medication, and synergistic efficacy with SARS-CoV-2-related vaccines.
When using pharmacotherapies to treat COVID-19, the severity of the disease plays a significant role in deciding which medications to use [15]. In Pang et al, the authors used bevacizumab to treat patients with severe COVID-19, as identified by their respiratory status and supplemental oxygen utilization (from nasal cannula to positive pressure ventilation) [16]. In our case, our patient had moderate COVID-19, defined as “individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have oxygen saturation ≥ 94% on room air” [15]. The patient never deteriorated after receiving bevacizumab during the duration of his hospitalization (no need for supplemental oxygen, no super-imposed bacterial infections). Such an emphasis is appropriate given he had risk factors for deterioration, from his radiographic imaging showing ground glass opacities to his age to his pre-existing co-morbidities [3]. Therefore, as bevacizumab continues to build evidence for its use in patients hospitalized with COVID-19, attention is warranted to using the pharmacotherapy early in the disease spectrum in order to prevent severe COVID-19.
Another consideration of the advantage of intravenous bevacizumab is its safety and limited adverse reactions. In Pang et al, their dose utilized for bevacizumab was on the lower end (7.5 mg/kg) [16], as bevacizumab dosing ranges from 5 to 15 mg/kg. Our patient received 5 mg/kg, which is the standard dose for patients receiving intravenous bevacizumab for HHT-related complications [11, 17, 18]. This lower dose should be considered as it provides substantial clinical benefits for patients with HHT and is well tolerated. The main side effects of the medication in patients with HHT appear to be transient hypertension and proteinuria [19], both of which self-resolve with the medication discontinuation. Thromboembolic events have also been reported with the use of bevacizumab, though it is unclear if it is directly due to the medication alone or when bevacizumab is used in certain conditions that raise the risk of a thromboembolism (e.g., with other thromboembolic-causing agents) [20]. Recognizing that the patients in Pang et al’s study were in severe COVID-19, with already established severe COVID-19 and its resulting systemic consequences [16], it is difficult to ascertain if the side effects found in the 27 patients receiving bevacizumab were medication-related or due to their systemic illness. However, if bevacizumab finds its most effective utility in COVID-19 is with moderate COVID-19, this well-tolerated pharmacotherapy at 5 mg/kg for patients with HHT may find similar tolerance in patients with moderate COVID-19.
A confounder in our case report as compared to Pang et al is the COVID-19-related treatments, from remdesivir to SARS-CoV-2 vaccine status, as Pang et al’s participants were recruited before these interventions were readily available. As for remdesivir, in the context of several clinical trials now published, the current evidence to support its use in patients hospitalized with mild to moderate COVID-19 has fallen to a BIIa (B = moderate, IIa = other randomized control trials or subgroup analyses of randomized control trials) [15]. With vaccine status, the patient received an mRNA vaccine, which has continued to show efficacy in preventing severe COVID-19 during the pandemic [21, 22]. Therefore, as the pandemic continues and vaccine rates rise, many current pharmacotherapies, as well as ones being explored, may have to show their efficacy in preventing and/or surviving severe COVID-19 in conjunction with SARS-CoV-2-related vaccines and other COVID-19-specific pharmacotherapies. In our patient’s case, recognizing the limitation of a case report, it is not entirely certain how much bevacizumab contributed to the patient’s recovery from COVID-19 in the setting of his vaccine status and remdesivir. Regardless, if pharmacotherapies have the ability to be more effective in the setting of vaccines and other medications, then they should continue to be explored as there will still be a significant amount of patients warranting hospitalization for COVID-19 and in need of tolerable interventions to assist in halting disease progression.
In conclusion, our case report demonstrates the potential utility of low-dose intravenous bevacizumab in patients with moderate COVID-19. Building on current evidence of the efficacy and tolerability of bevacizumab in patients with severe COVID-19 [16], such a pharmacotherapy should continue to be explored in larger trials to assure there is a clinical benefit from the intervention. Currently, there is one active study recruiting patients evaluating bevacizumab in patients with severe hypoxemia from COVID-19 (NCT04822818). Further, given the antiangiogenic properties of bevacizumab, it may find use in other conditions whereby VEGF has an active role in pathogenesis and/or disease progression, paralleling its expansion from oncological benefit into HHT-related benefit. Our case should continue to reaffirm the benefit of repurposing pharmacotherapies whose target(s) often transcend various diseases, thereby, providing benefit across these diverse disease states.
Learning points
While COVID-19 continues to be a global public health crisis, pharmacotherapies that result in effective COVID-19 management will continue to be sought. Pharmacotherapies that target aberrant immune factors may be attractive as they factor in preventing severe COVID-19 and/or promoting survival. Bevacizumab, a monoclonal antibody with VEGF inhibition properties, has accumulating evidence to support its use in severe COVID-19. Our case demonstrates that intravenous bevacizumab may also have a role in moderate COVID-19 and at the lowest possible dosing regimen (5 mg/kg), findings that warrant ongoing investigations.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no financial disclosures.
Conflict of Interest
The authors declare they have no conflict of interest.
Informed Consent
Informed consent was obtained from the patient for this presentation.
Author Contributions
RPF performed the necessary background investigation, and summarized all references and discussion points. NRR, CRW, and SS assisted in the write-up of the manuscript. PG oversaw the genesis of the manuscript and editing. PG and SS cared for the patient and provided the case summary. All authors contributed in editing the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239-1242.
doi pubmed - Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
doi - Chidambaram V, Tun NL, Haque WZ, Majella MG, Sivakumar RK, Kumar A, Hsu AT, et al. Factors associated with disease severity and mortality among patients with COVID-19: A systematic review and meta-analysis. PLoS One. 2020;15(11):e0241541.
doi pubmed - Jin Y, Ji W, Yang H, Chen S, Zhang W, Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5(1):293.
doi pubmed - Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389-391.
doi pubmed - de Gussem EM, Kroon S, Hosman AE, Kelder JC, Post MC, Snijder RJ, Mager JJ. Hereditary Hemorrhagic Telangiectasia (HHT) and survival: the importance of systematic screening and treatment in HHT centers of excellence. J Clin Med. 2020;9(11):3581.
doi pubmed - Faughnan ME, Mager JJ, Hetts SW, Palda VA, Lang-Robertson K, Buscarini E, Deslandres E, et al. Second international guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. Ann Intern Med. 2020;173(12):989-1001.
doi pubmed - Li S, Wang SJ, Zhao YQ. Clinical features and treatment of hereditary hemorrhagic telangiectasia. Medicine (Baltimore). 2018;97(31):e11687.
doi pubmed - Al-Samkari H, Kritharis A, Rodriguez-Lopez JM, Kuter DJ. Systemic bevacizumab for the treatment of chronic bleeding in hereditary haemorrhagic telangiectasia. J Intern Med. 2019;285(2):223-231.
doi pubmed - Sadick H, Naim R, Gossler U, Hormann K, Riedel F. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int J Mol Med. 2005;15(1):15-19.
doi pubmed - Al-Samkari H, Kasthuri RS, Parambil JG, Albitar HA, Almodallal YA, Vazquez C, Serra MM, et al. An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: the InHIBIT-Bleed study. Haematologica. 2021;106(8):2161-2169.
- Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, Millar T, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med. 2021;203(2):192-201.
doi pubmed - Sahebnasagh A, Nabavi SM, Kashani HRK, Abdollahian S, Habtemariam S, Rezabakhsh A. Anti-VEGF agents: As appealing targets in the setting of COVID-19 treatment in critically ill patients. Int Immunopharmacol. 2021;101(Pt B):108257.
doi pubmed - Epperla N, Kapke JT, Karafin M, Friedman KD, Foy P. Effect of systemic bevacizumab in severe hereditary hemorrhagic telangiectasia associated with bleeding. Am J Hematol. 2016;91(6):E313-314.
doi pubmed - Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda (MD), 2021.
- Pang J, Xu F, Aondio G, Li Y, Fumagalli A, Lu M, Valmadre G, et al. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nat Commun. 2021;12(1):814.
doi pubmed - Albitar HAH, Almodallal Y, Gallo De Moraes A, O'Brien E, Choby GW, Pruthi RK, Stokken JK, et al. Intravenous bevacizumab in hereditary hemorrhagic telangiectasia-related bleeding and high-output cardiac failure: significant inter-individual variability in the need for maintenance therapy. Mayo Clin Proc. 2020;95(8):1604-1612.
doi pubmed - Albitar HAH, Almodallal Y, Papadakis KA, Rajan E, Kamath PS, Iyer VN. Intravenous bevacizumab reduces transfusion requirements and endoscopic interventions in patients with gastric antral vascular ectasia and small bowel angioectasia. Gastroenterology. 2020;158(4):1162-1163.e1164.
doi pubmed - Guilhem A, Fargeton AE, Simon AC, Duffau P, Harle JR, Lavigne C, Carette MF, et al. Intra-venous bevacizumab in hereditary hemorrhagic telangiectasia (HHT): A retrospective study of 46 patients. PLoS One. 2017;12(11):e0188943.
doi pubmed - Alahmari AK, Almalki ZS, Alahmari AK, Guo JJ. Thromboembolic events associated with bevacizumab plus chemotherapy for patients with colorectal cancer: a meta-analysis of randomized controlled trials. Am Health Drug Benefits. 2016;9(4):221-232.
- Pilishvili T, Gierke R, Fleming-Dutra KE, Farrar JL, Mohr NM, Talan DA, Krishnadasan A, et al. Effectiveness of mRNA COVID-19 Vaccine among U.S. Health Care Personnel. N Engl J Med. 2021;385(25):e90.
doi pubmed - Thomas SJ, Moreira ED, Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761-1773.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


