| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 7, July 2022, pages 330-334
Unusual Presentation of Isolated Nonbacterial Thrombotic Tricuspid Valve Endocarditis in Systemic Lupus Erythematosus With Secondary Antiphospholipid Syndrome: A Case Report
Talwinder Nagia, f, Nitasa Sahub, Natasha Usmanic, Anil Ramad, Jamshed Zuberie
aDepartment of Internal Medicine, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
bDepartment of Pulmonary and Critical Care, St. Lukes University Health Network, Bethlehem, PA 18015, USA
cDepartment of Community Medicine, Shalamar Medical and Dental College, Lahore, Pakistan
dStanford Medical Center, Palo Alto, CA 94304, USA
eDepartment of Trauma Surgery, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
fCorresponding Author: Talwinder Nagi, Department of Internal Medicine, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
Manuscript submitted May 4, 2022, accepted June 23, 2022, published online July 20, 2022
Short title: Unusual Presentation of NBTE in SLE With APLS
doi: https://doi.org/10.14740/jmc3950
| Abstract | ▴Top |
Nonbacterial thrombotic endocarditis (NBTE), or marantic endocarditis, is one of the most prevalent cardiac presentations seen in patients with systemic lupus erythematosus (SLE). It is a condition that is characterized by noninfectious lesions affecting cardiac valves. The most common sight for this disease to affect is the left-sided mitral and aortic cardiac valves. It rarely involves the right-sided tricuspid valve. However, having a secondary condition that increases risk for hypercoagulability can potentiate the severity and frequency of cardiac valvular disease in SLE. In this report, the authors describe a rare case of a patient who presented with clinically symptomatic isolated-sterile tricuspid valve vegetations likely due to antiphospholipid syndrome (APLS) on top of SLE. Optimal medical and surgical managements of these vegetations are not well defined. Criteria call for surgical intervention in infective endocarditis when there are severe heart failure or valve dysfunction, prosthetic valve infection, recurrent systemic emboli, large mobile vegetations, and other detrimental complications. However, intervention for sterile vegetations should also be discussed if the patient can benefit from it clinically and if it can improve quality of life. The authors discuss this case in the context of the relevant medical and surgical literature.
Keywords: Nonbacterial thrombotic endocarditis; Tricuspid valve; Systemic lupus erythematosus; Antiphospholipid syndrome; Sterile vegetations
| Introduction | ▴Top |
Nonbacterial thrombotic endocarditis (NBTE), or marantic endocarditis, is a disease characterized by the presence of sterile vegetations on one or more cardiac valves. This means that no organisms or signs of inflammation are found in these vegetations or the blood stream at the time of clinical presentation. NBTE has increasingly been recognized as an entity associated with other conditions such as malignancy, hypercoagulable states, and autoimmune diseases like systemic lupus erythematous (SLE) and antiphospholipid syndrome (APLS). NBTE mainly involves the aortic and mitral valves, and when compared to infective endocarditis, the vegetations are considered more easily removed from the surface of valve leaflets [1]. This report presents a patient with symptomatic NBTE secondary to hypercoagulability from underlying autoimmune conditions. This case is unique since the tricuspid valve was affected instead of the more common left-sided valves. The patient was found to have moderate-to-severe tricuspid valve regurgitation due to the size of the sterile vegetations. The importance of this case is to discuss the possible need for surgical intervention for patients with severe regurgitation. For example, standard criteria for infectious endocarditis consider surgical intervention appropriate once signs of severe heart failure or recurrent systemic emboli become evident. Despite there being no standard criteria for sterile right-sided vegetations, surgery could help prolong the non-symptomatic period and improve the quality of life for this patient.
| Case Report | ▴Top |
Investigations
A 44-year-old female patient presented to the emergency department complaining of shortness of breath and right-sided chest pain starting 2 days prior. She denied ever having similar symptoms to this severity previously. The pain was described as sharp, stabbing, and 8/10 in intensity with no further radiation. The pain was worse with moderate exertion and alleviated by leaning forward. She could not recline past 45° and walking more than 15 steps made her feel short of breath. The patient complained of chronic right lower leg swelling, for which she would use two pillows to elevate her legs when trying to sleep. She also mentioned having symmetric joint stiffness of her wrists for the last 6 months that would improve throughout the day. She denied headaches, visual changes, auditory changes, dry mouth, fevers, chills, diaphoresis, dysphagia, abdominal pain, back pain, urinary changes, and bowel changes. Her medical history was notable for heavy menstrual periods, a miscarriage at age 14, and a history of chronic deep vein thrombosis (DVT) refractory to warfarin. Her last DVT was more than 2 years ago. She had a surgical history of a non-retrievable inferior vena cava (IVC) filter placement in 2018, breast reduction in 2003, and tummy tuck procedure in 2010. The patient did not take any prescribed medications but had been taking ibuprofen as needed for pain and had a history of oral contraceptive use which she discontinued prior to her IVC filter placement. For family history, the patient mentioned hypertension in her mother and type 2 diabetes mellitus along with hypertension in her father. She was unaware of any other health conditions that ran in her family. The patient is a stay-at-home mother and denied history of smoking, alcohol use, illicit drug use, and intravenous drug use. She frequently travels to the Dominican Republic and mentioned having an episode of mild right-sided chest pain during her last visit in early March 2020, for which the doctor there told her she did not need treatment.
On exam, she was an obese female, slightly tachypneic, sitting forward in her bed. Her blood pressure was 116/91 mm Hg, 98% oxygen saturation on room air, and afebrile. She had a mild nonpruritic, non-tender rash located on her nose and cheeks. The patient clarified that she had previous episodes of a similar rash that would subside on its own. Other pertinent findings on the physical exam included a jugular venous distension, a tender chest wall upon palpation, and a grade 2/6 pan-systolic murmur at the left sternal border that was more pronounced on inspiration. She had bibasilar Velcro sounds upon auscultation of the lungs.
Diagnosis
To further assist in diagnosing this patient, a series of laboratory and imaging investigations were ordered. A complete blood count was ordered to assess for signs of anemia, infection, or thrombosis. This patient had a low hemoglobin of 10.2 g/dL and a platelet count of 71,000,000/mm3, which can support an image of thrombocytopenia. Her erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were both elevated at 55 mm/h and 90.9 mg/L, respectively, suggesting presence of an inflammatory process. Her D-dimer was elevated at 3.7 µg/mL, therefore indicating the need of a computed tomography (CT) angiography of the chest and lower extremity duplex ultrasound to rule out possible DVT and pulmonary embolism, especially given her medical history. Duplex ultrasound showed no sonographic evidence of DVT in her right femoral vein and the left femoral vein was patent. The CT angiography of the chest showed small bilateral pleural effusions with multifocal areas of atelectasis and was negative for any pulmonary embolism. The pleural effusion found could be due to overload from impaired right-sided heart function, which could also explain the positive jugular vein distention (JVD). Her routine chemistry, urinalysis, toxicology, hepatitis panel, and blood culture were insignificant.
On coagulation workup, the partial thromboplastin time (PTT) was elevated at 49 s with a normal prothrombin time (PT), international normalized ratio (INR), and fibrinogen level. The electrocardiogram (EKG) showed normal sinus rhythm with low-voltage QRS. Chest X-ray showed cardiomegaly, blunting of the right costophrenic angle, and elevated diaphragm bilaterally. A completed echocardiogram showed a normal ejection fraction of 55-60%. The left side of the heart was unremarkable. However, the right side had moderate-to-severe tricuspid regurgitation, a 1.6 × 0.9 cm tricuspid valve vegetation, and mild pericardial effusion. Based on these results, further evaluation with a transesophageal echocardiogram (TEE) was warranted and found all three tricuspid valve leaflets with vegetations on the ventricular surface. The anterior leaflet vegetation was 1.51 × 1.06 cm in measurement (Fig. 1). The septal leaflet was 1.05 × 1.33 cm and the posterior leaflet was 0.76 × 1.03 cm (Fig. 2). Based on the clinical picture, physical exam, and extensive workup, rheumatic heart disease or NBTE were considered as the differentials.
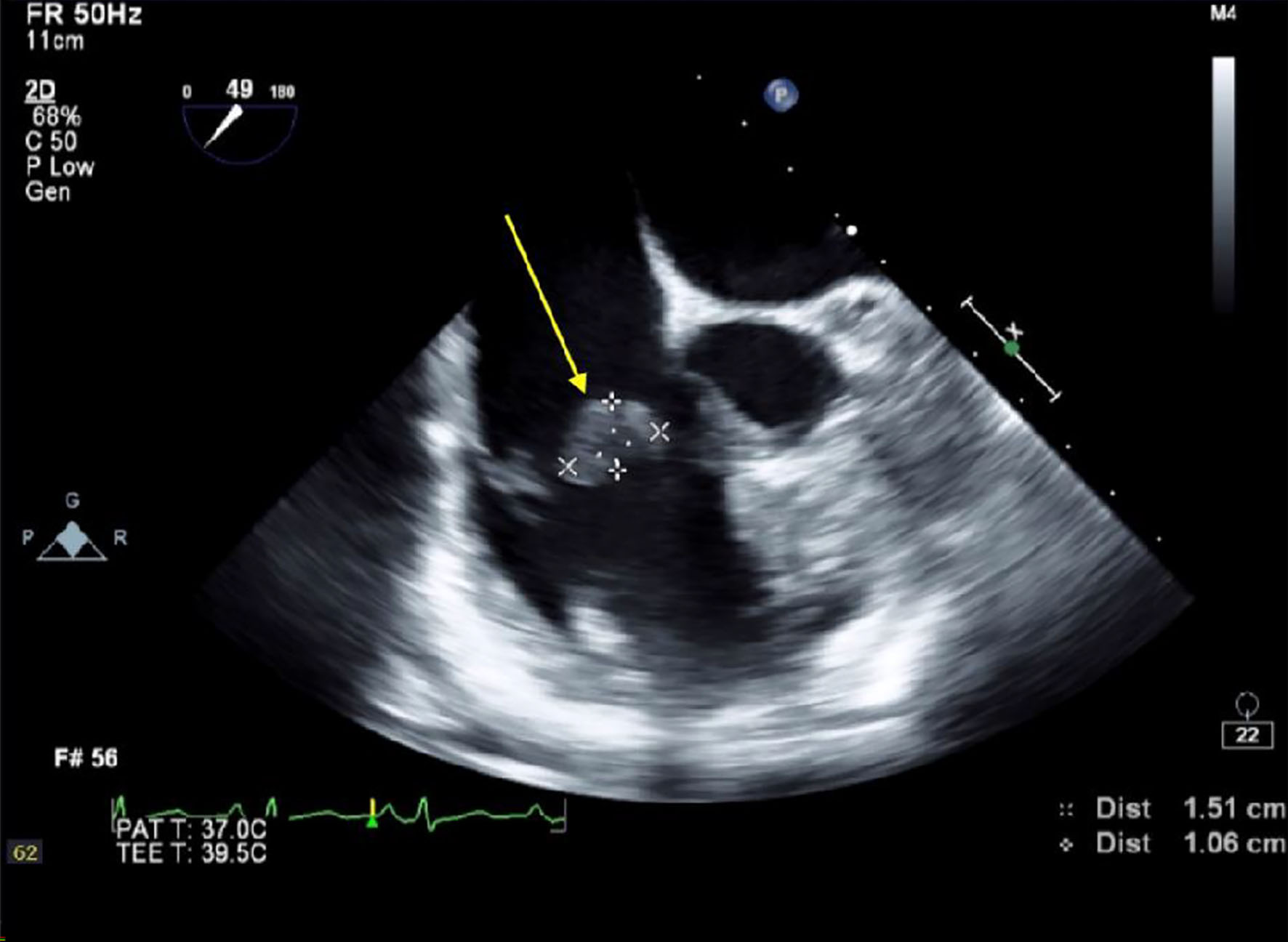 Click for large image | Figure 1. Transesophageal echocardiogram (TEE) completed at St. Joseph’s University Medical Center, Paterson, NJ. Arrow depicts vegetation on the anterior leaflet of the tricuspid valve measuring 1.51 × 1.06 cm. |
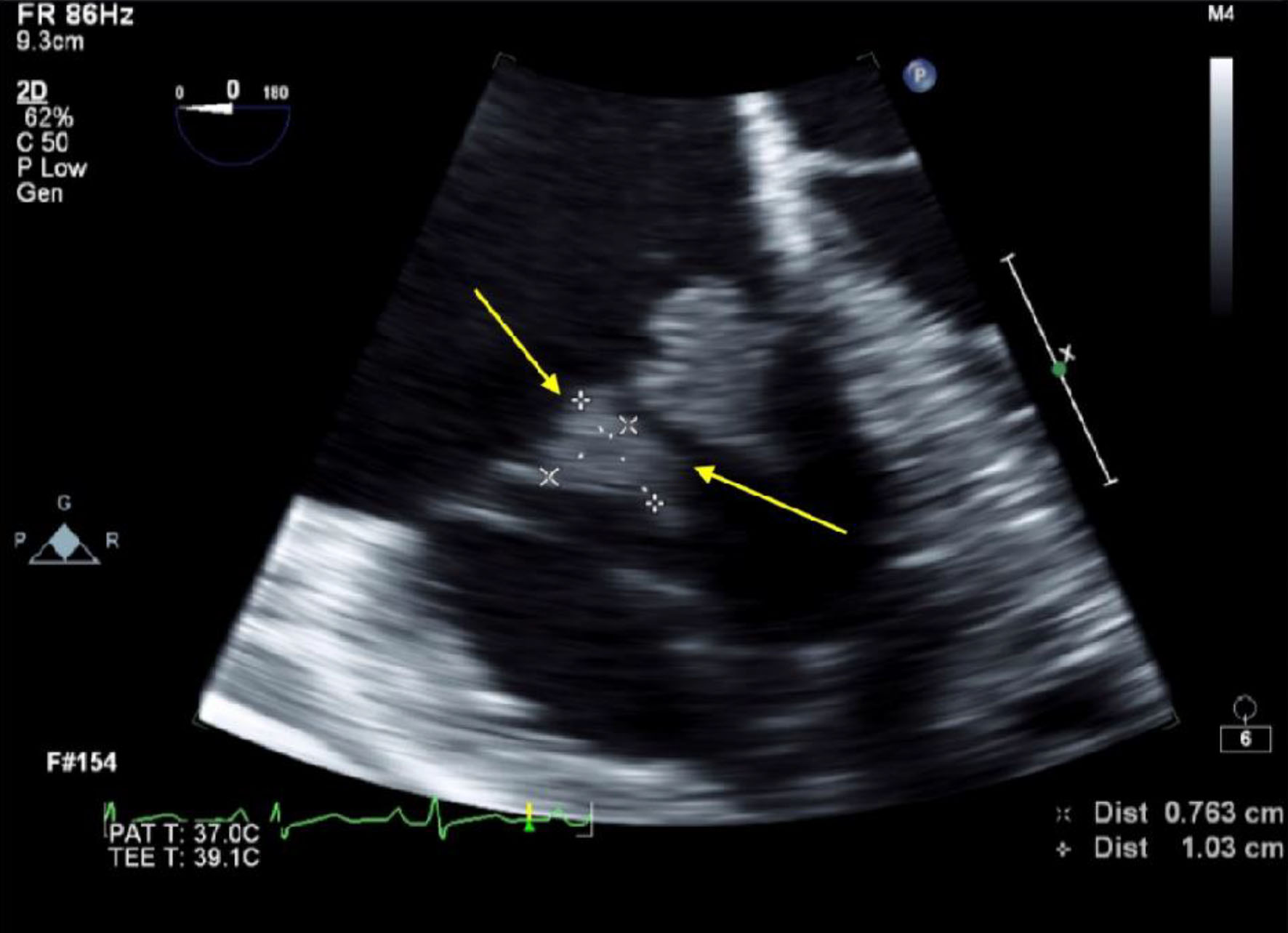 Click for large image | Figure 2. Transesophageal echocardiogram (TEE) completed at St. Joseph’s University Medical Center, Paterson, NJ. Arrow depicts vegetation on the posterior leaflet of the tricuspid valve measuring 0.76 × 1.03 cm. |
Results of this patient’s serological panel helped to understand the etiology of her symptoms. The first result was the lupus anticoagulant which was positive. This gives her a diagnosis of APLS. This diagnosis can explain her presentation of recurrent DVTs and a history of miscarriage. It is also supported by findings on her coagulation workup since lupus anticoagulant can cause a falsely elevated PTT. Her anti-double-stranded DNA antibody came back positive which is a sign of concurrent SLE. RNP antibodies for the patient were also positive, suggesting a picture of mixed connective tissue disorder. Her anti-SSA and SSB antibodies for Sjogren syndrome were also elevated. We examined her immunoglobulin and complement levels which showed increased IgG, IgA and low C4, respectively. This finding suggests a possibility of type III hypersensitivity where consumption of complement occurs through formation of immune complexes which deposit in the body. Overall, the diagnosis of NBTE due to SLE with secondary APLS was made.
Treatment
The patient was placed on therapeutic lovenox, ibuprofen, colchicine, and hydroxychloroquine. After initiation of these medications, the patient’s clinical symptoms of positional chest pain improved. Since the tricuspid valve vegetations were large and causing symptomatic moderate-to-severe regurgitation, surgical intervention was discussed with cardiothoracic surgery. However, due to the patient’s normal ejection fraction and lack of other concrete signs of severe heart failure and recurrent embolisms, a decision was made to continue medical management and no intervention was necessary at the time.
Follow-up and outcomes
With resolution of symptoms, the patient was discharged and told to follow-up outpatient with cardiology. At the first follow-up appointment, the patient was directed to continue hydroxychloroquine and meloxicam for SLE and continued to be asymptomatic with no chest pain or shortness of breath. Warfarin was also continued for APLS and her recurrent DVTs. At a subsequent appointment with cardiology, the patient complained of mild shortness of breath on exertion and an echocardiography was completed. The ejection fraction remained unchanged from her initial one done during admission. It was advised that the patient continue her prescribed medications and monitor her symptoms. Suppose the patient has a recurrence of chest pain and shortness of breath with a more deteriorated clinical presentation. In that case, we believe it would be important to consider the implications of surgical intervention for her valvular vegetations despite not meeting the general standard criteria.
| Discussion | ▴Top |
NBTE is characterized by deposition of sterile platelet thrombi onto cardiac valves. These cardiac vegetations are non-infectious and can be seen in certain disease states such as acute stroke, coronary ischemia, SLE, APLS, and malignancy. Patients with advanced malignancy and SLE are the most common populations affected [2]. Symptoms associated with NBTE have a varying degree of presentation. Patients often present with no symptoms and cardiac murmurs are usually not appreciated [2]. Murmurs can be heard when the disease progresses in about 50% of cases [2]. However, symptoms are more prominent when vegetations begin to embolize to other organs in the body such as the skin, spleen, kidney, and extremities. Presence of systemic emboli can lead to symptomatic NBTE in up to 50% of patients compared to solely having valvular dysfunction [3]. When valvular dysfunction is observed, it commonly presents with left-sided valve impairment. In a study done on 171 cases of NBTE, left-sided vegetations were most common with 64% on the mitral valve, 24% on the aortic, and 9% presenting with vegetations on both [4]. Vegetations affecting the right-sided tricuspid valve are very rare and not appreciated in many studies [4].
The pathogenesis of NBTE affecting valves is not fully understood, but mechanisms such as hypercoagulability and immune complex deposition are implicated in the initiation of the disease. The commonality of the two is production of endothelial damage, exposing the subendothelial tissue to circulating platelets [2]. In Libman-Sacks endocarditis, a form of NBTE seen in some with SLE, there is formation of fibrin-platelet thrombi on the cardiac valve which leads to fibrosis and eventual valvular scaring and dysfunction. The thrombi in this disease are unique in that they usually deposit on both surfaces of the left-sided heart valves and are typically sessile, small, and wart-like [2]. However, vegetations seen in APLS present as valve masses or thickening [5]. APLS is an autoimmune condition characterized by venous or arterial thrombosis, history of pregnancy loss, and presence of either anticardiolipin antibodies, anti-beta2-glycoprotein l antibodies, or lupus anticoagulant. This syndrome can occur alongside SLE and increases the risk for manifestations of hypercoagulability. Presence of antiphospholipid antibodies in SLE is associated with a significant risk of heart valve disease [5]. The risk is more pronounced in those with SLE and positive lupus anticoagulant, such as seen in our patient [6].
Venous thromboembolism is a hallmark of APLS and can explain the history of recurrent DVTs and miscarriage in our patient. Since venous veins are responsible for carrying blood back to the heart, it is possible for any thrombus formed in the venous circulation to get carried to the heart and deposited onto heart valves. However, our patient does have an IVC filter in place which is supposed to prevent passage of venous thrombi into the lungs leading to possible pulmonary embolism. The pathogenesis of the vegetations present in this patient is likely due to her secondary APLS which increases the chances of cardiac involvement in SLE patients. It also increases hypercoagulability and along with the immune complex formations, is enough to lead to endothelial damage of the valves and exposure to fibrin and platelet complexes. The presentation of the vegetations on the tricuspid valve and only affecting the ventricular surface, makes it unique when compared to the usual findings in NBTE.
Since the vegetations in this patient lead to symptomatic tricuspid regurgitation and signs of right-sided overload, it is necessary to explore the need for surgical intervention through tricuspid valve replacement.
For right-sided infective endocarditis, the indications for surgery are not well-defined but should be considered in patients with tricuspid valve vegetations greater than 20 mm and recurrent septic pulmonary emboli with or without right heart failure, infective endocarditis caused by organisms that are hard to eradicate or bacteremia for at least 7 days, or right heart failure secondary to severe tricuspid regurgitation with poor response to diuretics [7]. For NBTE affecting the tricuspid valve, indications and timing for surgery have not been formally discussed [8]. However, in general, it is essentially the same for infective endocarditis and includes uncontrollable heart failure or infectious and mobile vegetations [8]. Even though our patient was relatively stable, her vegetations are large and it could put her at an increased risk for pulmonary embolism and stroke. If surgery is not sought out, management with anticoagulation and treating her underlying condition is put in place and continued long term. Heparin or warfarin are the mainstay for managing the hypercoagulability; however, few studies have shown that it does not fully prevent future emboli leaving the patient still at risk for complications [8, 9]. Thus, we propose that patients not clinically presenting with the need for surgical intervention should be followed with close clinical and echocardiographic monitoring. If further symptomatic periods occur that affect a patient’s quality of life, surgical intervention should still be considered despite not meeting any prior published indication for endocarditis intervention.
Learning points
Overall, surgical intervention should be considered for patients with moderate-to-severe regurgitation due to sterile cardiac vegetations. Medical management with heparin or warfarin has been the mainstay for managing hypercoagulability in patients with SLE with affected cardiac valves. However, it has not been shown to fully prevent emboli from occurring in the future. Despite there being no standard criteria for sterile right-sided vegetations, surgery could help prolong the non-symptomatic period and improve the quality of life for the patient.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
The manuscript has been de-identified. Informed consent was obtained from the patient.
Author Contributions
TN wrote the case report, participated in the patient care, revised, and edited the case report, and approved the final version of the case report. NS, NU, AR, and JZ revised and edited the case report and approved the final version of the case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Seki A, Fishbein MC. Chapter 2 - Age-related cardiovascular changes and diseases. In: Buja LM, Butany J (eds). Cardiovascular pathology (Fourth Edition). Academic Press. 2016:57-83.
doi - Asopa S, Patel A, Khan OA, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007;32(5):696-701.
doi pubmed - el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12(5):518-523.
doi pubmed - Steiner I. [Nonbacterial thrombotic endocarditis—a study of 171 case reports]. Cesk Patol. 1993;29(2):58-60.
- Hojnik M, George J, Ziporen L, Shoenfeld Y. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation. 1996;93(8):1579-1587.
doi pubmed - Zuily S, Regnault V, Selton-Suty C, Eschwege V, Bruntz JF, Bode-Dotto E, De Maistre E, et al. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: meta-analysis of echocardiographic studies. Circulation. 2011;124(2):215-224.
doi pubmed - Hussain ST, Witten J, Shrestha NK, Blackstone EH, Pettersson GB. Tricuspid valve endocarditis. Ann Cardiothorac Surg. 2017;6(3):255-261.
doi pubmed - Kaneyuki D, Matsuura K, Ueda H, Kohno H, Kanbe M, Matsumiya G. Surgical management of nonbacterial thrombotic endocarditis in malignancy. Surg Case Rep. 2017;3(1):60.
doi pubmed - Borowski A, Ghodsizad A, Cohnen M, Gams E. Recurrent embolism in the course of marantic endocarditis. Ann Thorac Surg. 2005;79(6):2145-2147.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


