| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 7, July 2022, pages 354-358
Ortner’s Syndrome in an Infant With Congenital Heart Disease
Marcelino Murillo-Deluqueza, Christopher McKeea, b, Misael Collazos-Noriegac, Clifford L. Cuad, Joseph D. Tobiasa, b, e
aDepartment of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA
bDepartment of Anesthesiology & Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
cDepartment of Anesthesiology, Clinica General del Norte, Barranquilla, Colombia
dDivision of Pediatric Cardiology, Department of Pediatrics, Nationwide Children’s Hospital, The Ohio State University, Columbus, OH, USA
eCorresponding Author: Joseph D. Tobias, Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH 43205, USA
Manuscript submitted May 12, 2022, accepted June 23, 2022, published online July 20, 2022
Short title: Ortner’s Syndrome With CHD
doi: https://doi.org/10.14740/jmc3959
| Abstract | ▴Top |
Cardio-vocal or Ortner’s syndrome is dysphonia or hoarseness resulting from left recurrent laryngeal nerve palsy caused by a mechanical effect on the nerve due to enlarged cardiovascular or mediastinal structures. It was first described in adults with left atrial enlargement due to mitral stenosis. To date, there are a paucity of reports regarding its occurrence in infants and children. We report hoarseness and left vocal cord paresis in an infant with a large left-to-right shunt associated with a patent ductus arteriosus. The history of Ortner’s syndrome is presented, its pathogenesis described, and previous reports of its occurrence in infants and children reviewed.
Keywords: Ortner’s syndrome; Cardio-vocal syndrome; Patent ductus arteriosus; Hoarseness; Vocal cord paralysis; Left recurrent nerve paralysis
| Introduction | ▴Top |
Hoarseness or dysphonia is generally described as a change in the quality of the voice or cry. The voice or cry may be described as raspy, strained, or weak [1]. There may be an associated change in the pitch, restriction of range or decreased projection of the voice [2]. These changes can be caused by any process that affects the structure or function of the larynx and surrounding structures including the vocal cords. Cardio-vocal or Ortner’s syndrome is hoarseness due to left recurrent laryngeal nerve palsy caused by a mechanical effect or stretching of the nerve due to enlarged cardiovascular or mediastinal structures, generally occurring in adults [3-5]. We describe hoarseness due to left vocal cord paresis in an infant with significant left atrial and pulmonary artery enlargement due to a large left-to-right shunt associated with a patent ductus arteriosus (PDA). The history of Ortner’s syndrome is presented, its pathogenesis described, and previous reports of its occurrence in infants and children reviewed.
| Case Report | ▴Top |
Investigations
Review of this case and presentation in this format followed the guidelines of the Institutional Review Board of Nationwide Children’s Hospital (Columbus, OH, USA). The patient was a 5-month-old, 5.4-kg female infant with a past medical history of failure to thrive and mild vesicoureteral reflux/hydronephrosis. Pregnancy was complicated by maternal Wolff-Parkinson-White syndrome with supraventricular tachycardia treated with flecainide, hypertension treated with labetalol, as well as anxiety and depression treated with sertraline. Prenatal imaging consisted of a fetal magnetic resonance imaging (MRI) and fetal echocardiogram demonstrating a ductal aneurysm and a non-stenotic dysplastic pulmonary valve. Labor and delivery were uncomplicated.
Diagnosis
An echocardiogram on the first day of life showed persistence of the PDA and ductal aneurysm with left-to-right shunting, a thickened pulmonary valve, trivial (physiologic) pulmonary valve regurgitation, a small secundum atrial septal defect (ASD), mild right ventricular hypertension, and normal biventricular size and systolic function. There was a left aortic arch with normal branching and no evidence of a vascular ring. The remainder of the neonatal course was uncomplicated, and the neonate was discharged home with mother with cardiology follow-up for the PDA.
Treatment
Following hospital discharge, there were concerns regarding poor weight gain due to feeding issues and recurrent respiratory infections. These issues led to planning for PDA closure. Prior to the planned surgical ligation of the PDA, the infant was admitted to the hospital at 4 months of age for treatment of respiratory insufficiency due to respiratory syncytial virus (RSV) bronchiolitis, which progressed to multifocal pneumonia. After admission to the pediatric intensive care unit (ICU), the infant was noted to have a weak cry with concerns for intermittent stridor and choking with feeds. Respiratory failure required endotracheal intubation and mechanical ventilation for 12 days. Following tracheal extubation, noninvasive support was required with high-flow nasal cannula (HFNC) and bilevel positive airway pressure (BiPAP) due to increased work of breathing and tachypnea. Enteral nutrition was provided by nasogastric or nasoduodenal feeds. Due to a persistent weak cry and concerns for stridor, nasoendoscopy was performed by the pediatric otolaryngology service which revealed left vocal cord paresis. Repeat echocardiogram showed increasing left-to-right shunting, a moderately large PDA, severe dilatation of the pulmonary arteries and left atrial enlargement (Fig. 1). After recovery from the viral illness, at 5 months of age, the infant was taken to the operating room for direct laryngoscopy and ligation of the PDA. Direct laryngoscopy confirmed left vocal cord paralysis with failure of full adduction of the left vocal cord resulting in a glottic gap. The vocal cord was injected with aqueous glycerin carboxymethylcellulose gel implant (Prolaryn™ gel) to facilitate glottic closure and prevent aspiration during feeding. Due to the size of the PDA, ligation via a thoracotomy was not feasible. The PDA was ligated via a median sternotomy during cardiopulmonary bypass for 54 min. The procedure was uneventful, and the infant was admitted postoperatively to the cardiothoracic ICU. Her trachea was extubated in the operating room following the surgical procedure. Postoperative respiratory support was provided with BiPAP or HFNC for 48 h and then weaned to nasal cannula and eventually room air. Postoperative hypertension was treated with sodium nitroprusside that was transitioned to enteral enalapril when she was tolerating oral medications. The remainder of her postoperative course was uneventful.
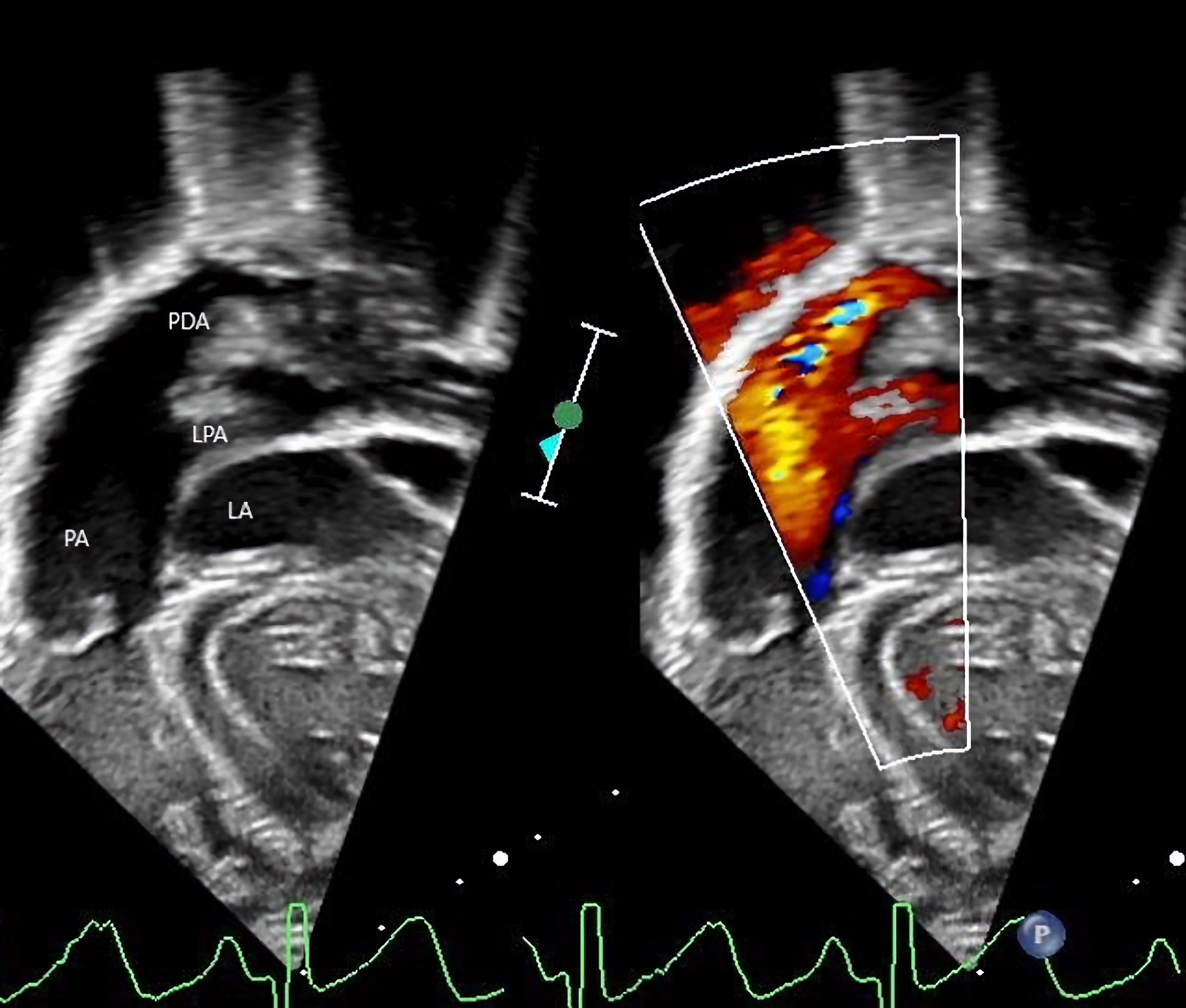 Click for large image | Figure 1. Echocardiogram showing persistence of the patent ductus arteriosus (PDA), ductal aneurysm with left-to-right shunting, and left atrial (LA) enlargement. LPA: left pulmonary artery; PA: pulmonary artery. |
Follow-up and outcomes
Postoperatively, there was no stridor noted and the infant was eventually able to feed without aspiration or choking. Three months after the injection laryngoplasty, flexible fiberoptic nasoendoscopy revealed normal vocal cord function and movement with good glottic closure.
| Discussion | ▴Top |
The recurrent laryngeal nerves provide motor innervation to the intrinsic laryngeal muscles for vocalization and glottic opposition or adduction to prevent aspiration. The recurrent laryngeal nerves are branches of the vagus nerve which has its origin in the medulla oblongata. The right and left recurrent laryngeal nerves are branches of the vagus nerve that descend into the neck and thorax. The right recurrent laryngeal nerve branches at the level of the right subclavian artery and hooks around this artery. The left recurrent laryngeal branches from the vagus nerve, travels caudad, and loops from an anterior to posterior position around the arch of the aorta (Fig. 2). It then ascends in the tracheoesophageal groove, anterior to the trachea and posterior to the aortic arch and the left lobe of the thyroid, where it enters the larynx and innervates the musculature of the glottis. As it travels under the aortic arch, it passes through a limited triangular space formed by the ligamentum arteriosum (PDA), aortic arch, and left pulmonary artery. At this point, the nerve can be compressed by enlargement of any of the structures as was likely the case in our patient. The space in the area known as the aortic triangle has been estimated to be as little as 4 mm in adults [6]. As such, the nerve is vulnerable at this point (Fig. 1).
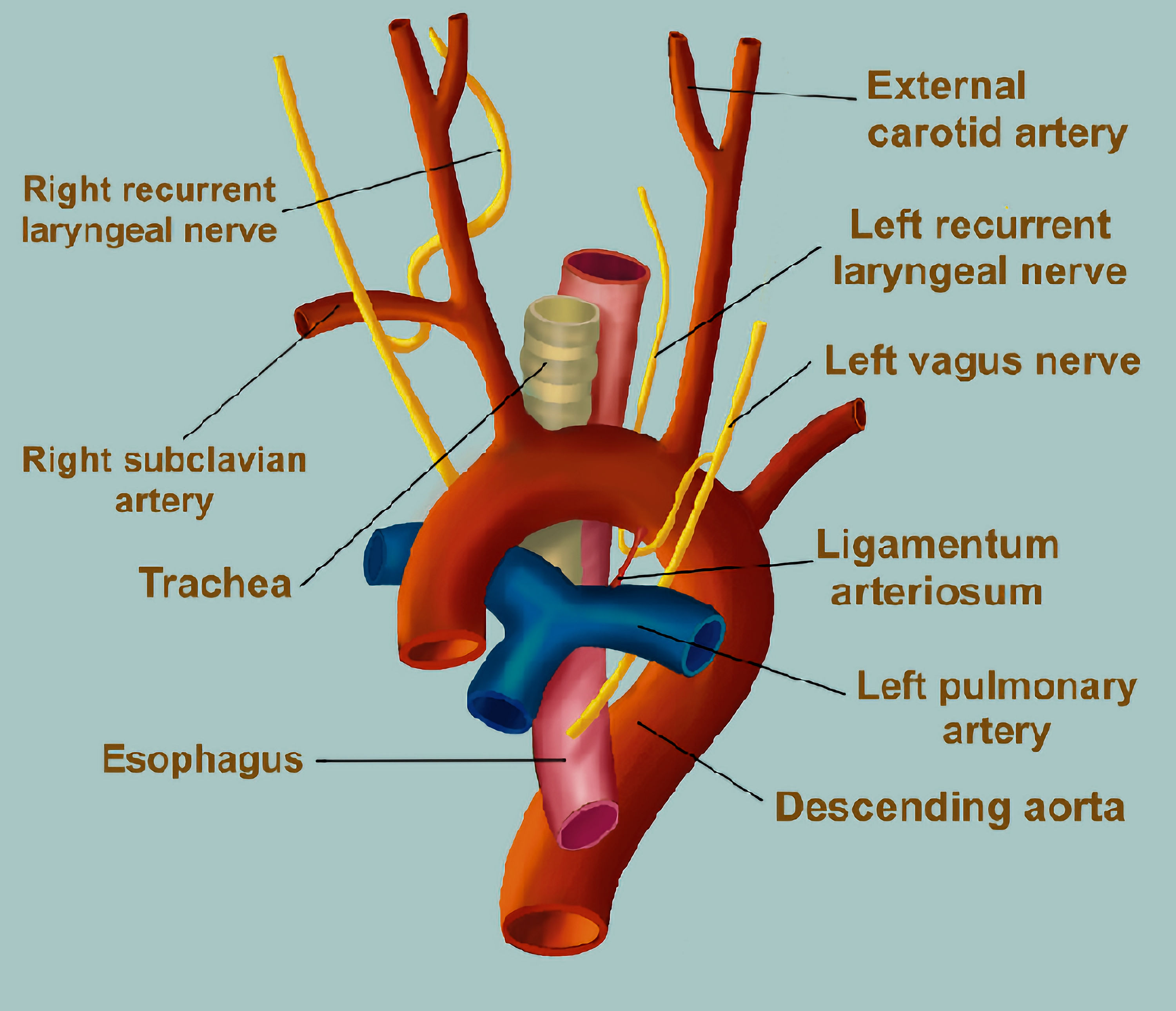 Click for large image | Figure 2. Diagram showing the course of the left recurrent laryngeal nerve as it branches from the left vagus nerve and travels under the arch of the aorta. It passes through the aortic triangle formed by the ligamentum arteriosum (patent ductus arteriosus), aortic arch, and left pulmonary artery. |
The first description of vocal paralysis in association with cardiovascular pathology was by Norbert Ortner, an Austrian physician in 1897. Dr. Ortner attributed left vocal cord immobility due to recurrent laryngeal nerve compression by a dilated left atrium in association with mitral valve stenosis [7]. He postulated that the enlarged left atrium directly compresses the left recurrent laryngeal nerve against the aortic arch. Others have refuted these findings suggesting that the primary pathophysiologic mechanism must involve pulmonary artery dilatation and enlargement. In 1911, Fetterolf and Norris postulated that the recurrent laryngeal nerve must be compressed between the left pulmonary artery and the aortic arch or the ligamentum arteriosum [8]. Further support for this pathophysiologic mechanism was provided by autopsy findings in 1934 by King et al [9]. Degeneration of the left recurrent laryngeal nerve was identified at the point where the recurrent laryngeal nerve passes in the space bordered by the left pulmonary artery, the arch of the aorta, and the ductus arteriosus in patients with vocal cord paralysis and left ventricular failure. Stocker et al coined the term “cardio-vocal syndrome” noting the occurrence of left vocal cord paralysis with heart disease [10]. However, the exact pathophysiology remains debatable the presence of a dilated pulmonary artery is rarely associated with vocal cord paralysis. Therefore, other etiologic factors have been proposed to play an accompanying role including mediastinitis, pericardial effusion, obliterative pericarditis, pleural effusion, and lymphadenitis [11].
In adults, cardio-vocal syndrome has been reported most commonly in association with acquired or congenital cardiac disease including mitral stenosis, left ventricular failure, primary pulmonary hypertension, thoracic aneurysms, and PDA [6, 10, 12-14]. In infants and children, cardio-vocal syndrome has been most commonly reported in association with congenital heart disease (CHD) including PDA, double-outlet right ventricle atrial septal defect, ventricular septal defect (VSD), thoracic aneurysms, surgical repair of coarctation of the aorta, and Ebstein anomaly or pulmonary hypertension [3, 4, 15-17].
Accurate diagnosis requires a high index of suspicion with clinical signs including voice changes, dysphonia, hoarseness, or stridor in a patient with CHD or pulmonary hypertension. As noted in our patient, the clinical signs may be subtle including only stridor or mild voice changes. Additionally, as noted in our patient, as adduction of the vocal cord is affected, feeding difficulties with chronic aspiration may be noted. The incidence is increased in CHD associated with pulmonary artery dilatation as can be seen with a large left-to-right shunt. In adults, laryngeal electromyography has been used to evaluate vocal cord paralysis, confirm the diagnosis, and define the etiology [18, 19]. However, in infants and children, otolaryngology evaluation with flexible or rigid airway evaluation is generally indicated thereby demonstrating left vocal cord paresis. Treatment options include surgical repair of the underlying CHD which may correct the left-to-right shunt and vascular enlargement compressing the recurrent laryngeal nerve. Surgical intervention including injection of the vocal cord may improve adduction and limit aspiration. In our patient, PDA ligation and resolution of the pulmonary artery dilatation likely relieved the pressure on the recurrent laryngeal nerve and allowed for resolution of vocal cord paralysis. However, as the vocal cord was also injected at the time of the PDA ligation, it may be that there is not a direct causal effect with resolution of left vocal cord paralysis as a result of the PDA ligation and resolution of pulmonary artery dilatation.
Learning points
Vocal cord paralysis, otherwise known as Ortner’s or cardio-vocal syndrome, was first described in an adult patient with mitral stenosis. Since then, it has been described in both adults and children with enlargement of cardiovascular structures due to acquired heart disease or CHD with impingement on the left recurrent laryngeal nerve as it passes through a triangular space formed by the ligamentum arteriosum (PDA), aortic arch, and left pulmonary artery. Clinical signs include voice change or stridor in patients with associated cardiovascular disease or CHD. Feeding difficulties with chronic aspiration may be noted in infants. Treatment is generally aimed at the associated congenital or acquired heart disease.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from a parent for anesthetic care and potential publication. The patient information was de-identified for publication.
Authors Contributions
MMD performed the initial case review and manuscript preparation, literature review, and editing of subsequent revisions. CM provided clinical care and reviewed the manuscript. MCN provided Figure 2 and reviewed the manuscript. CLC provided clinical care for the patient, provided echocardiographic images and labels, and reviewed the manuscript. JDT contributed to literature review, manuscript writing, and editing of the manuscript.
Data Availability
The data supporting the findings of this case report are available from the authors.
| References | ▴Top |
- Cohen SR, Thompson JW, Geller KA, Birns JW. Voice change in the pediatric patient. A differential diagnosis. Ann Otol Rhinol Laryngol. 1983;92(5 Pt 1):437-443.
doi pubmed - Hirschberg J, Dejonckere PH, Hirano M, Mori K, Schultz-Coulon HJ, Vrticka K. Voice disorders in children. Int J Pediatr Otorhinolaryngol. 1995;32(Suppl):S109-125.
doi - Subramaniam V, Herle A, Mohammed N, Thahir M. Ortner's syndrome: case series and literature review. Braz J Otorhinolaryngol. 2011;77(5):559-562.
doi pubmed - Zaki SA, Asif S, Shanbag P. Ortner syndrome in infants. Indian Pediatr. 2010;47(4):351-353.
doi pubmed - Nambiar R, Dalus D, Srikumar A. Cardiovocal Syndrome: A rare cause of hoarseness in a patient with a history of pulmonary tuberculosis. Sultan Qaboos Univ Med J. 2017;17(4):e481-e483.
doi pubmed - Nakahira M, Nakatani H, Takeda T. Left vocal cord paralysis associated with long-standing patent ductus arteriosus. AJNR Am J Neuroradiol. 2001;22(4):759-761.
- Ortner N. Recurrenslahmung bei Mitralstenose. Wien Klin Wochenschr. 1897;10:753-755.
- Fetterolf G, Norris GW. The anatomical explanation of the paralysis of the left recurrent laryngeal nerve found in certain cases of mitral stenosis. Am J Med Sci. 1911;141:625-638.
doi - Cagnol C, Garcin M, Unal D. [A case of severe congenital laryngeal stridor treated by hyomandibulopexy]. J Fr Otorhinolaryngol Audiophonol Chir Maxillofac (1967). 1971;20(4):625-626.
- Stocker HH, Enterline HT. Cardio-vocal syndrome: laryngeal paralysis in intrinsic heart disease. Am Heart J. 1958;56(1):51-59.
doi - Hillman LM, Malcomson K. Ortner's syndrome. Guys Hosp Rep. 1956;105(3):307-319.
- Yasui T, Kasamatsu N, Seto T, Shinozuka N, Nakamura A, Hashizume I. [A case of Ortner syndrome caused by primary pulmonary hypertension]. Nihon Kokyuki Gakkai Zasshi. 2006;44(11):823-827.
- Hermans C, Manocha S, McLaughlin JE, Lipman M, Lee CA. Ortner syndrome and haemophilia. Haemophilia. 2005;11(5):548-551.
doi pubmed - Chan P, Lee CP, Ko JT, Hung JS. Cardiovocal (Ortner's) syndrome left recurrent laryngeal nerve palsy associated with cardiovascular disease. Eur J Med. 1992;1(8):492-495.
- Zaki SA, Banur D. Ortner's syndrome as a presenting feature of congenital heart disease in infants. Heart Views. 2020;21(2):118-120.
doi pubmed - Robida A, Povhe B. Cardiovocal syndrome in an infant with a double outlet of the right ventricle. Eur J Pediatr. 1988;148(1):15-16.
doi pubmed - Kaya O, Yoldas T, Karademir S, Orun UA, Sari E. A pediatric case of Ortner's syndrome caused by heritable pulmonary arterial hypertension and review of the literature. Turk J Pediatr. 2019;61(6):963-966.
doi pubmed - Akbulut S, Inan R, Demir MG, Cakan D. Laryngeal electromyography is helpful for cardiovocal syndrome. Acta Medica (Hradec Kralove). 2016;59(1):29-32.
doi pubmed - Gavazzoni FB, Scola RH, Lorenzoni PJ, Kay CS, Werneck LC. The clinical value of laryngeal electromyography in laryngeal immobility. J Clin Neurosci. 2011;18(4):524-527.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


