| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 9, September 2022, pages 443-448
Novel Treatment of Ventilator Dyssynchrony From Central Alveolar Hypoventilation Syndrome Utilizing Scheduled 5-Hydroxytryptamine-3 Receptor Antagonist
Aldin Malkoca, b, Ashley Stadinga, Stephanie Wonga, Tara Weavera, Leslie Ghislettaa
aDepartment of General Surgery, Arrowhead Regional Medical Center, Colton, CA 92324, USA
bCorresponding Author: Aldin Malkoc, Department of General Surgery, Arrowhead Regional Medical Center, Colton, CA 92324, USA
Manuscript submitted July 19, 2022, accepted August 17, 2022, published online September 28, 2022
Short title: Novel Treatment of Ventilator Dyssynchrony
doi: https://doi.org/10.14740/jmc3983
| Abstract | ▴Top |
Traumatic brain injury (TBI) occurs in a large percentage of surgical trauma patients and is one of the leading causes of death amongst young teens and adults. Furthermore, individuals with TBIs often require mechanical ventilation and admission to the intensive care unit. As a result of their TBIs, these patients can develop central alveolar hypoventilation (CAH) secondary to disruptions in neuromodulatory respiratory brainstem control and neural signal initiation and integration. Prior studies have primarily focused their attention on treatment of congenital disorders of CAH, and limited research is available on intubated trauma patients who have signs of ventilator dyssynchrony. Current case reports and animal studies have suggested that noradrenergic and specific serotonergic medications are able to target specific neurologic pathways in the respiratory circuit and induce ventilator synchrony. This case series describes the clinical course of TBI patients treated for ventilator dyssynchrony secondary to CAH with a daily scheduled 5-hydroxytryptamine-3 (5-HT3) receptor antagonist. All patients were ultimately extubated and discharged from the hospital.
Keywords: Traumatic brain injury; Central alveolar hypoventilation; Ventilator; Ondansetron
| Introduction | ▴Top |
Traumatic brain injury (TBI) is a leading cause of death and disability among children and young adults in the United States [1]. A recent study by Wunsch et al reported that on average, 39.5% of patients admitted to the intensive care unit (ICU) receive mechanical ventilation at any given hour in the United States [2]. TBIs in multi trauma patients can cause disruption of the central respiratory system leading to prolonged mechanical ventilation and increased stay in the ICU [3, 4]. More specifically, individuals with TBIs develop central alveolar hypoventilation (CAH) disorders, which can be a result of conditions such as ischemia, tumors, neurodegenerative disorders, infection, and trauma [5-8]. CAH has been found to arise from disruptions affecting the sensors, the central controller, or the integration of neurologic signals within the central respiratory centers in the pons and medulla [3].
A high density of central nervous system 5-hydroxytryptamine-3 (5-HT3) receptors (serotonin receptors) are in brainstem areas, including trigeminal nucleus, the nucleus of the solitary tract (NTS), and area postrema regions that are involved in ascending somatosensory circuits to higher-order processing centers in the cortex (insula, and somatosensory) [9]. Ondansetron is a 5-HT receptor antagonist that decrease the serotonin activity in these regions. Ondansetron has a recognized inhibitory effect on vomiting especially through inhibitory effects on afferent terminals of the vagus nerve and nucleus tractus solitarius (NTS). Substantial effect is suspect on ventilatory control since ondansetron has been shown by Stern et al to reduce activation of insula and sensorimotor cortical regions which have been implicated in dyspnea sensation and respiratory sensory integration [10, 11].
Patients with TBIs will have impaired arousal requiring mechanical ventilation to protect their airway. Often patients with TBIs tend to develop ventilator asynchronies requiring prolonged mechanical intubation and considerations for earlier tracheostomies [3]. While etiologies of CAH may be inherited or acquired, current research predominantly focuses on cases of inherited CAH with few studies discussing treatments for acute acquired CAH secondary to trauma. This case series describes three patients with TBIs, who developed CAH secondary to their TBI with mechanical ventilator asynchrony as noted by ineffective respiratory effort and were treated with scheduled ondansetron and subsequently achieved ventilator synchrony and reduced ventilation requirements.
| Case Reports | ▴Top |
Case 1
A 26-year-old female with a past medical history of a motor vehicle accident 6 months prior that resulted in a TBI and seizures, presented to our trauma bay following another motor vehicle accident involving a semi-truck. While in the trauma bay, the patient exhibited gasping breathing with a Glasgow coma scale (GCS) of 8. She was subsequently intubated, sedated and fluid resuscitation with normal saline was started. An extended focused assessment with sonography for trauma (eFAST) showed questionable lung sliding on the patient’s right side and free fluid in the right hepatorenal recess. A right chest tube was placed, and the patient was taken to the operating room for an exploratory laparotomy. Intraoperatively, the patient was found to have mesenteric bleeding as well as a bucket handle injury to the small bowel and ascending colon, both of which were left in discontinuity. A Bogota bag was placed over the patient’s abdomen and an ABThera was placed. The patient was taken to the surgical intensive care unit (SICU) for hemodynamic monitoring.
Once stable, the patient was taken back to the operating room on hospital day 3 for re-exploratory laparotomy with small bowel anastomoses and right colon end-to-end anastomoses. Imaging studies were delayed due to the extent of the patient’s initial injuries. Imaging studies at day 4 revealed left basal ganglia hemorrhages (Fig. 1). Due to the high likelihood of requiring long-term ventilation, the patient had a percutaneous tracheostomy placed on hospital day 7. While the patient’s GCS improved to 11 during intubation, one of the major difficulties faced was weaning the ventilator settings. Prior to the patient’s abdominal closure her ventilator settings included pressure control-assist control (PC-AC) with a respiratory rate (RR) of 14, peak inspiratory pressure (PIP) of 24 and positive-end-expiratory-pressure (PEEP) of 8 on 25% fractional inspired oxygen (FiO2), and she would trigger apnea ventilation when placed on continuous positive airway pressure (CPAP) during spontaneous breathing trials (SBT).
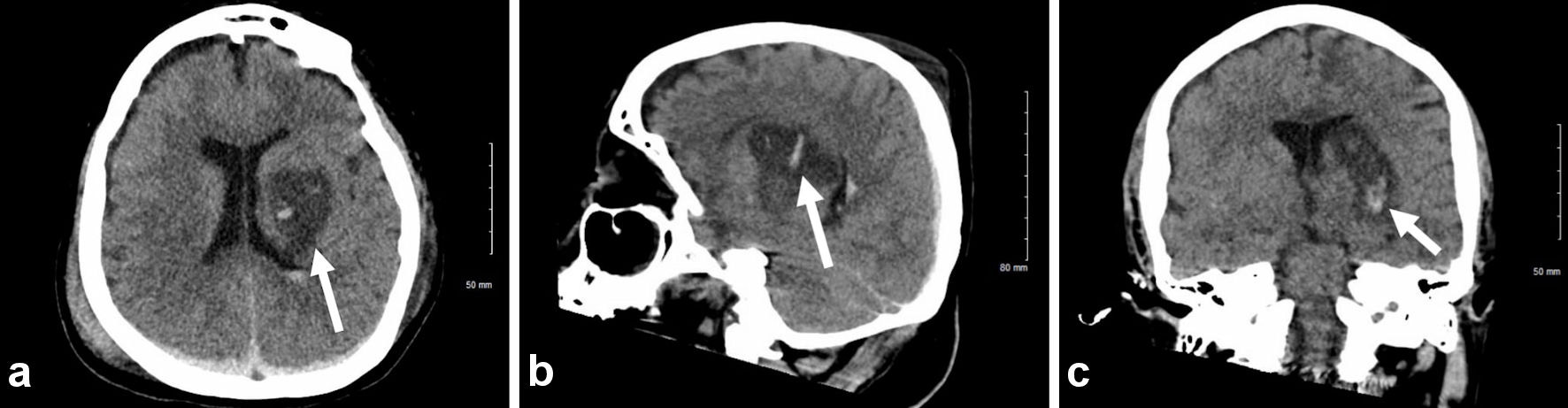 Click for large image | Figure 1. Axial non-contrast head CT with 5 mm slices showing bilateral subarachnoid (noted by white arrow) and intraparenchymal hemorrhages. CT: computed tomography. |
After the patient was placed on a tracheostomy, she was taken off all sedative medication and given lorazepam 0.5 mg intravenous (IV) every 2 h for agitation and multimodal pain control involving scheduled gabapentin, methocarbamol, Tylenol, and as needed oxycodone and IV morphine. The ventilator settings continued to be weaned to CPAP/pressure support (PS) and during weaning the patient would develop periods of apneic triggering when placed on CPAP with a PS of 8, PEEP 5, and FiO2 of 35%. At this time the patient was placed on pressure control-pressure support volume (PC-PSV) with RR of 12, PIP of 20, PEEP of 5, and FiO2 of 30%. The patient would continue to have episodes of apnea when placed on CPAP/PS requiring full rate on the ventilator. Post-TBI seizures were considered as an explanation for the apneic episodes, but the patient followed commands during the apneic episodes including taking a breath during a period of apnea she would. Additionally, during the apneic episodes the patient would not develop tachycardia but rather show episodes of bradycardia which was consistent with a vagal stimulus. In a patient with a prior TBI and new head trauma there was concern for central hypoventilation syndrome.
The patient was started on scheduled ondansetron 4 mg IV every 6 h on hospital day 13. By hospital day 16, the patient began to tolerate CPAP PS without any evidence of discomfort or apneic episodes for 120 min and on the same day the patient was placed on an Aerotrach at 35% FiO2 with SpO2 at 99% and no distress noted. On hospital day 23, the patient was on Aerotrach and was discharged to a long-term acute care facility with right sided hemiparesis and ondansetron was discontinued on discharge.
Follow-up and outcomes
Approximately 120 days after discharge the patient was called by the team to discuss her clinical course. It was reported she had a GCS of 15, had completed speech therapy, and was tolerating a regular diet. The patient no longer had a tracheostomy and has only mild residual right sided hemiparesis.
Case 2
A 70-year-old male with a past medical history of hypertension was brought in by air transport after suffering a fall from a 6-foot ladder. Per report by emergency medical services, the patient initially had a GCS of 3 that improved to 8 on route. While in the trauma bay, the patient deteriorated to a GCS of 6, and was emergently intubated and sedated. A computed tomography (CT) scan of the patient’s head revealed a parafalcine subdural hemorrhage (SDH), a right tentorial SDH, and a trace subarachnoid hemorrhage at the frontal vertex (Fig. 2). The remainder of the trauma survey also revealed a right tension pneumothorax requiring chest tube placement, fractures of the right fourth, 10th, and 11th ribs, a right pelvic hematoma, bilateral superior and inferior pubic rami fractures, a right sacrum fracture, and right anterior column fracture with involvement of the quadrilateral plate. The patient was admitted to the SICU for external ventricular drain placement and intracranial pressure monitoring.
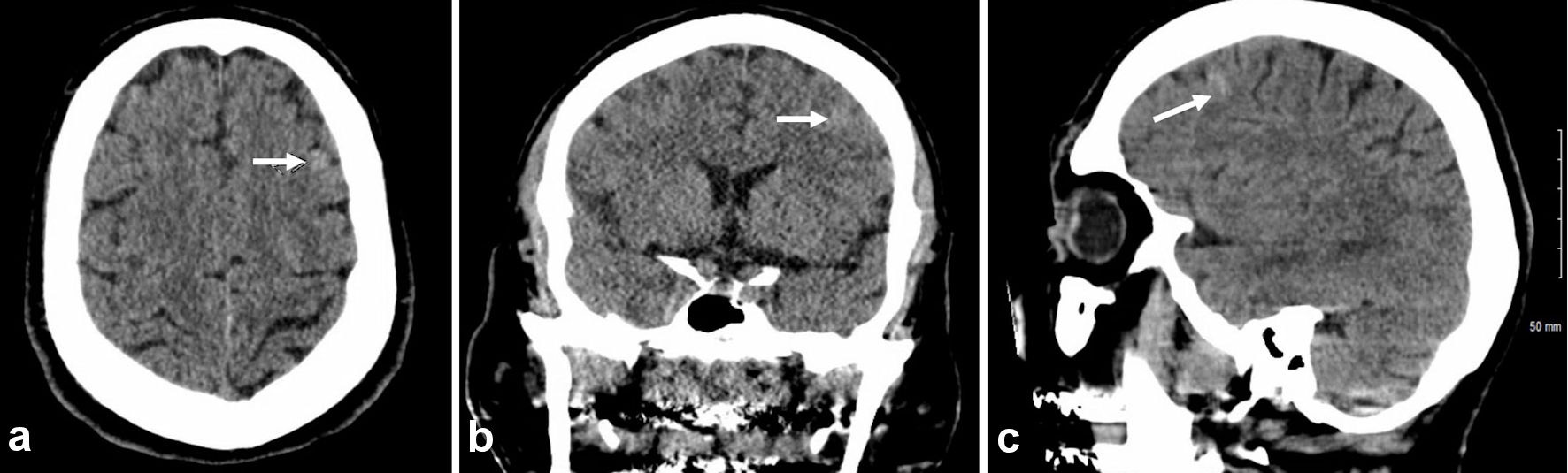 Click for large image | Figure 2. Axial non-contrast head CT with 5 mm slices with noted subdural blood along the right tentorium and posterior falx and small foci of subarachnoid blood (noted by white arrow) at the left frontal vertex. CT: computed tomography. |
On hospital day 4, the patient had improvement in neurologic status and his external ventricular drain was removed. Continuous electroencephalogram (EEG) on the same day showed slow wave activity with no evidence of seizures. By hospital day 8 the patient began exhibiting episodes of sympathetic hyperactivity and was started on propranolol and propofol for improved sedation. Due to the patient’s slow neurological improvement and apneic episodes when placed on CPAP during SBTs, magnetic resonance imaging (MRI) on hospital day 10 was performed and showed diffuse axonal injury. The patient subsequently had a tracheostomy placed on hospital day 11. Concern for central hypoventilation syndrome was suspected due to the patient experiencing repeated daily apneic episodes when placed on CPAP/PS. The patient was subsequently started on 4 mg ondansetron IV every 6 h. By hospital day 15, the patient was stable on PC-PSV with a RR of 12, PIP of 18, PEEP of 5, and FiO2 of 30%. At this time the patient began tolerating SBT and continuous sedative medications were transitioned to intravenous lorazepam as needed along with multimodal pain control. He did not exhibit signs of respiratory distress and was discharged to a long-term acute care facility.
Follow-up and outcomes
Approximately 30 days post discharge the patient was able to open his eyes, move fingers, squeeze his family’s hand, and had improving left lower extremity movement.
Case 3
A 25-year-old male with no significant past medical history presented to the emergency department following a head on motorcycle accident with a pick-up truck. The patient hit the back of a pick-up truck going at high speeds, and his helmet broke in half anteriorly. On arrival to the trauma bay, the patient deteriorated to a GCS of 5, was in respiratory distress, and combative. The patient was intubated and sedated for ventilatory support. The patient required bilateral tube thoracostomies and soon after placement developed hypotension with systolic blood pressures in the 50s. The remainder of the trauma survey revealed multiple lacerations exposing muscles, gross deformity of the mandible, and significant other abrasions. Imaging studies (Fig. 3) showed a right frontal lobe hemorrhage, hemorrhagic contusions of the basal ganglia, and right frontoparietal subarachnoid hemorrhage. The patient was admitted to the SICU for hemodynamic and external ventricular drain monitoring.
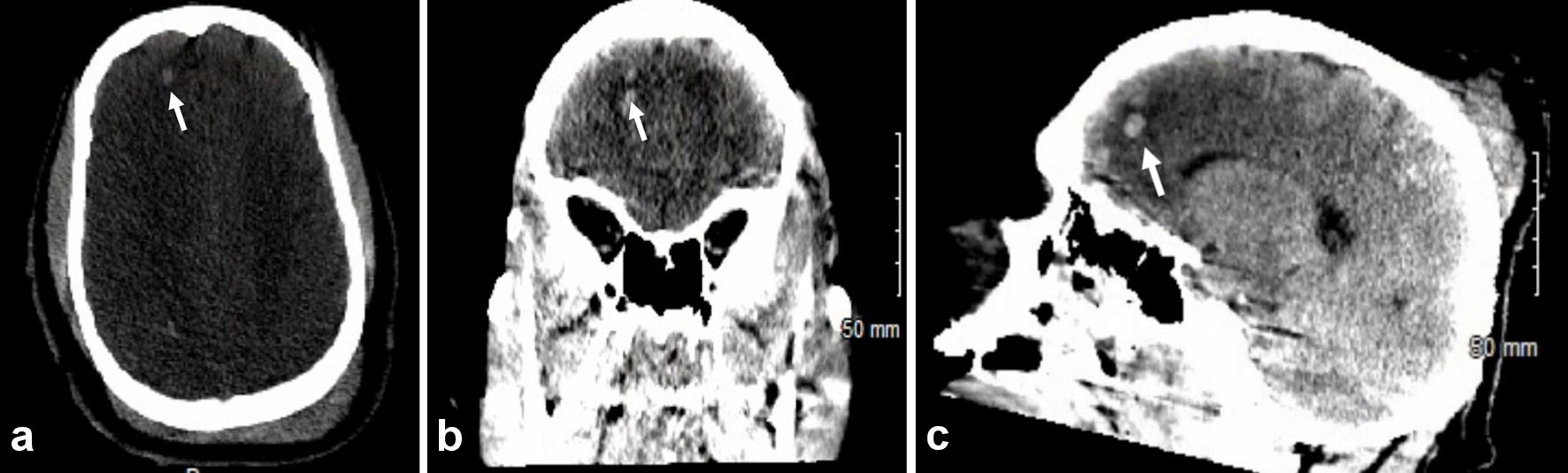 Click for large image | Figure 3. Axial non-contrast head CT with 5 mm slices showing scattered high densities in the right frontal lobe (noted by white arrow) and right basal ganglia consistent with contusion hemorrhages. Trace subarachnoid hemorrhages are also present in the right frontoparietal lobe. CT: computed tomography. |
By hospital day 3, a brain MRI showed grade II diffuse axonal injury. On hospital day 4 the patient developed decreasing RR with subsequent apnea and abnormal arterial blood gas findings during SBT. In the setting of a large TBI and suspected CAH the patient was started on 4 mg IV ondansetron every 6 h on hospital day 8, and the same day received a tracheostomy and percutaneous endoscopic gastrostomy tube. By hospital day 12 the patient had all continuous sedative medications removed and transitioned to lorazepam as needed and multimodal pain control as in case 1. At this time the patient was able to tolerate chest physiotherapy and daily SBTs when placed on CPAP with a PS of 8, PEEP 5, and FiO2 of 35%.
Follow-up and outcomes
The patient was subsequently discharged to an acute care facility and approximately 30 days after discharge the patient had begun to walk, talk, and regain memories.
| Discussion | ▴Top |
TBI refers to an injury sustained from an external force, effecting optimal brain function. Mechanisms of injury include direct impact, penetrating injury, and acceleration deceleration injuries. A serious complication of TBIs is central respiratory system disruption, which may lead to respiratory failure, requiring mechanical ventilation and ICU admission [12]. These injuries can lead to acquired CAH, a disorder caused by dysregulation of ventilatory control resulting in hypoxemia and hypercarbia. The disruptions caused by CAH can affect initiation, integration, or regulation of the respiratory impulse. Dysfunction in one or more of these phases leads to alterations in the rate or depth of respiration and presents as bradypnea, hypopnea, or in severe circumstances, apnea. Clinically, it can result in increases in carbon dioxide, decreases in oxygenation and leads to acid-base imbalance. This was noted in all three patients when attempting to wean the mechanical ventilator. It was not until patients were started on scheduled ondansetron improvement in mechanical ventilation was noted.
To understand the management of these disorders we first have to identify the common causes of CAH and understand how the respiratory impulse is propagated. CAH disorders are subclassified into three major categories: congenital, late-onset congenital, and acquired. Congenital CAH (CCAH) presents shortly after birth and is commonly referred to as Ondine’s curse; it is associated with mutations of the PHOX2B gene [13]. Although similar clinically, late-onset CCAH presents after 28 days of life. Both are marked by hypoventilation predominantly during non-rapid eye movement (NREM) sleep and by a proclivity for extended breath-holding. Supplemental oxygen is not sufficient in managing the course of this disease; tracheostomy and positive pressure ventilation is most appropriate for management, providing relief of hypercarbia. Current literature exhibits a more in depth understanding of CCAH compared with the acquired etiology. In acquired CAH, a neurologic insult to the central respiratory center such as ischemia, trauma, or inflammation leads to hypercapnia and hypoxemia.
The physiology of involuntary breathing is incredibly elaborate. Central nervous system control of normal physiological breathing involves the pontine and ventral respiratory groups, with specific neurons and central and peripheral chemoreceptors integrating various respiratory signals. The pontine respiratory group contains the pneumotaxic and apneustic center, providing constant feedback to the medulla to regulate respiratory rate and pattern. The pontine and ventral respiratory groups communicate with the apneustic center and provide excitatory input to the pre-Botzinger complex. The apneustic center is inhibited by the pneumotaxic center. Furthermore, sensory information from peripheral chemoreceptors and pulmonary mechanoreceptors is transmitted to the dorsal component of the ventral respiratory group for further integration and control of inspiration through the phrenic nerve. The caudal portion of the ventral respiratory group provides output signals to the expiratory muscles [14]. Arguably one of the most important sites of integration within the ventral respiratory group is the pre-Botzinger complex due to its initiation of the inspiratory process and acting as the respiratory center’s intrinsic pacemaker [15]. Harper et al determined that ventrolateral and dorsal medullary injury are damaged in different disease states of CAH [16]. This suggests that CAH affects the primary respiratory sites for chemosensing. As the patients reported in this study had no history of CAH prior to suffering a TBI, the timing and onset of their CAH can most likely be attributed to acquired neurological damage affecting the ventral respiratory group and its ability to integrate and transmit appropriate signals involved in ascending somatosensory circuits to higher-order processing centers in the cortex (insula, and somatosensory) [9]. Post-TBI seizures were considered as an explanation for the apneic episodes the patients developed, but the patients followed commands during them. With these episodes of apnea, the patients would also develop bradycardia, which was more consistent with a vagal stimulus.
Guyenet et al determined new integrations between the retrotrapezoid nucleus and serotonergic neurons located in the ventral respiratory group of the medulla have an intimate control of respiratory ventilation. Additionally, 5-HT exposure has been shown to enhance respiratory output [9], and inhibition of specific rhombomere 3/5-derived serotonergic neurons can attenuate ventilation [9]. In a mouse study by Potenzieri et al, it was shown that ondansetron administration was able to block apnea [17]. This effect was also described by Yoshioka et al in a rat study that determined ondansetron works by blocking the inhibitory afferent signal at the nodose ganglia of the vagus nerve that terminates at the ventral respiratory group [18]. The selective serotonergic antagonist is thought to block the inhibitory neurons that innervate integration sites within the ventral respiratory group and slow the intrinsic automaticity of the respiratory centers [19]. From the studies of Guyenet et al and prior animal studies there are few plausible explanations for the benefit of selective serotonergic antagonist in acquired CAH in the surgical trauma patient.
The treatment of the CCAH is better described in the literature than its acquired counterpart; however, as it is a rare genetic disease, the evidence is still sparse. Diagnosis and assessment of severity of disease is typically done via polysomnography both during sleep and wakefulness. After diagnosis, the aim of management is to avoid the deleterious effects of acute or chronic hypoxemia and hypercarbia on neurologic development and functioning. Management of CCAH primarily involves lifelong ventilatory support and close monitoring of the progression of autonomic dysregulation [20]. There are four main types of ventilatory support for CCAH patients: positive pressure ventilation via tracheostomy, mask ventilation, phrenic nerve pacing, and negative pressure ventilation. Each type is used for different reasons. Currently, many studies have explored various molecules that may help with ventilation, but no medication has been shown to improve ventilation sufficiently to sustain life without ventilatory support. For the purposes of this study, we will focus on the medications currently being studied and used in the context of both congenital and acquired CAH.
Several case reports and small prospective studies have reported some efficacy of various medications in treating CAH. These include acetazolamide (ACZ), carbamazepine, desogestrel, mirtazapine, and buspirone [21-24]. ACZ is a carbonic anhydrase inhibitor that causes mild metabolic acidosis and a subsequent increase in respiratory drive, which has been proposed as the mechanism behind decreasing the frequency of central sleep apnea in spinal cord injury patients [21]. There has been one case of a child with CCAH who was treated with carbamazepine and subsequently noted to have improved apneic episodes during waking hours, however not during sleep-related episodes. One proposed explanation for this clinical improvement was the ability of carbamazepine to inhibit the effect of adenosine, a known respiratory depressant, on its receptors [22]. Mirtazapine, an agonist at 5-HT1 receptors and antagonist at 5-HT2 and 5-HT3 receptors, was shown to significantly reduce the apnea index during sleep in rats with central apnea [23]. Buspirone, a serotonin receptor agonist, was shown in a study of eight patients with chronic spinal cord injury and central sleep apnea to decrease central sleep apnea [24]. Lastly, peripherally administered 5-HT has been shown to block apneic episodes [17]. Specifically, ondansetron is thought to block the inhibitory afferent impulses that originate at the nodose ganglia of the vagus nerve and terminate at the ventral respiratory group [18]. It is theorized that ondansetron blocks these inhibitory neurons that innervate integration sites within the ventral respiratory group and can ultimately reduce activation of specific centers such as the insula and sensorimotor cortical regions to reduce dyspnea sensation [10, 11].
Herein, we described three cases of patients with acquired CAH who initially had high ventilatory requirements but subsequently made a remarkable recovery after being treated with ondansetron. Further prospective studies would be the next step in exploring the relationship between ondansetron and decreasing ventilatory requirements and overall intubation time in patients with TBI.
Learning points
Trauma-acquired CAH is an understudied topic. The various processes of respiratory regulation are a complex topic. Due to the persistent apneic episodes noted in patients with TBI our use of ondansetron promoted ventilator synchrony. The patients were able to quickly come off the ventilator and continually show improvement in their rehabilitation.
Acknowledgments
The authors would like to thank and express their gratitude to the exceptional nursing staff and unit managers of the Arrowhead Regional Medical Center Intensive Care Unit for their expert clinical support.
Financial Disclosure
The authors have no financial or funding disclosures.
Conflict of Interest
The authors declare there is no conflict of interest.
Informed Consent
Written informed consent was obtained from the patients for publication of this case reports and any accompanying images. An institutional review board (IRB) approval was submitted and approved per Arrowhead Regional Medical Center. The patients were appropriately deidentified for this manuscript.
Author Contributions
AM performed the initial manuscript write-up, literature review, and editing of the manuscript. AS assisted with literature review and editing of the manuscript. SW assisted with initial manuscript write-up, literature review, and editing of the manuscript. TW assisted with initial manuscript write-up, literature review, and editing of the manuscript. LG attended on the cases and contributed to decision-making, management of the patient, literature review, as well as editing of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
TBI: traumatic brain injury; ICU: intensive care unit; CAH: central alveolar hypoventilation; CCAH: congenital CAH; NTS: nucleus of the solitary tract; GCS: Glasgow Coma Scale; SICU: surgical intensive care unit; eFAST: extended focused assessment with sonography for trauma; PC-AC: pressure control-assist control; RR: respiratory rate; PIP: peak inspiratory pressure; PEEP: positive-end-expiratory-pressure; PC-PSV: pressure control-pressure support volume; FiO2: fractional inspired oxygen; CPAP: continuous positive airway pressure; SBT: spontaneous breathing trials; SDH: subdural hemorrhage; NREM: non-rapid eye movement; ACZ: acetazolamide
| References | ▴Top |
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14(6):602-615.
doi pubmed - Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41(12):2712-2719.
doi pubmed - Dunham CM, Cutrona AF, Gruber BS, Calderon JE, Ransom KJ, Flowers LL. Early tracheostomy in severe traumatic brain injury: evidence for decreased mechanical ventilation and increased hospital mortality. Int J Burns Trauma. 2014;4(1):14-24.
- Abujaber A, Fadlalla A, Gammoh D, Abdelrahman H, Mollazehi M, El-Menyar A. Using trauma registry data to predict prolonged mechanical ventilation in patients with traumatic brain injury: Machine learning approach. PLoS One. 2020;15(7):e0235231.
doi pubmed - Muzumdar H, Arens R. Central alveolar hypoventilation syndromes. Sleep Med Clin. 2008;3(4):601-615.
doi pubmed - Nogues MA, Benarroch E. Abnormalities of respiratory control and the respiratory motor unit. Neurologist. 2008;14(5):273-288.
doi pubmed - Kim H, Bach JR. Central alveolar hypoventilation in neurosarcoidosis. Arch Phys Med Rehabil. 1998;79(11):1467-1468.
doi - Rao GS, Ramesh VJ, Lalla RK. Ventilatory management and weaning in a patient with central hypoventilation caused by a brainstem cavernoma. Acta Anaesthesiol Scand. 2005;49(8):1214-1217.
doi pubmed - Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron. 2015;87(5):946-961.
doi pubmed - von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Buchel C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med. 2008;177(9):1026-1032.
doi pubmed - Stern ER, Shahab R, Grimaldi SJ, Leibu E, Murrough JW, Fleysher L, Parides MK, et al. High-dose ondansetron reduces activation of interoceptive and sensorimotor brain regions. Neuropsychopharmacology. 2019;44(2):390-398.
doi pubmed - Centers for Disease Control and Prevention. Rates of TBI-related emergency department visits by age group-United States, 2001-2010. 2016. https://www.cdc.gov/traumaticbraininjury/data/rates_ed_byage.html.
- Lovell BL, Bullock RE, Anderson KN. An unusual presentation of congenital central hypoventilation syndrome (Ondine's Curse). Emerg Med J. 2010;27(3):237-238.
doi pubmed - Zaidi S, Gandhi J, Vatsia S, Smith NL, Khan SA. Congenital central hypoventilation syndrome: An overview of etiopathogenesis, associated pathologies, clinical presentation, and management. Auton Neurosci. 2018;210:1-9.
doi pubmed - Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36(3):152-162.
doi pubmed - Harper RM, Kumar R, Ogren JA, Macey PM. Sleep-disordered breathing: effects on brain structure and function. Respir Physiol Neurobiol. 2013;188(3):383-391.
doi pubmed - Potenzieri C, Meeker S, Undem BJ. Activation of mouse bronchopulmonary C-fibres by serotonin and allergen-ovalbumin challenge. J Physiol. 2012;590(21):5449-5459.
doi pubmed - Yoshioka M, Goda Y, Togashi H, Matsumoto M, Saito H. Pharmacological characterization of 5-hydroxytryptamine-induced apnea in the rat. J Pharmacol Exp Ther. 1992;260(2):917-924.
- Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;5:CD003002.
doi - Trang H, Samuels M, Ceccherini I, Frerick M, Garcia-Teresa MA, Peters J, Schoeber J, et al. Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J Rare Dis. 2020;15(1):252.
doi pubmed - Ginter G, Sankari A, Eshraghi M, Obiakor H, Yarandi H, Chowdhuri S, Salloum A, et al. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. J Appl Physiol (1985). 2020;128(4):960-966.
doi pubmed - Schirwani S, Pysden K, Chetcuti P, Blyth M. Carbamazepine improves apneic episodes in congenital central hypoventilation syndrome (CCHS) with a novel PHOX2B exon 1 missense mutation. J Clin Sleep Med. 2017;13(11):1359-1362.
doi pubmed - Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med. 1999;160(6):1824-1829.
doi pubmed - Maresh S, Prowting J, Vaughan S, Kruppe E, Alsabri B, Yarandi H, Badr MS, et al. Buspirone decreases susceptibility to hypocapnic central sleep apnea in chronic SCI patients. J Appl Physiol (1985). 2020;129(4):675-682.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


