| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 11, November 2022, pages 525-529
Psychosis and Bilateral Peripheral Facial Palsy Associated With COVID-19
Carla Jevouxa , Abouch Krymchantowskia
, Raimundo Pereira Silva-Netob, g
, Ervin Michelstaedter Cotrikc, d, Antonio Egidio Nardie, Joao Pedro Gomesf, Ana Gabriela Krymchantowskia
aDepartment of Neurology, Headache Center of Rio, Rio de Janeiro, Brazil
bDepartment of Neurology, Federal University of the Parnaiba Delta, Parnaiba, Brazil
cDepartment of Psychiatry, Centro de Psicologia Aplicacao e Formacao, Rio de Janeiro, Brazil
dUniversidade de Santiago de Compostela, Spain
eInstitute of Psychiatry, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
fDepartment of Neurology, Hospital Geral de Bom Sucesso, Rio de Janeiro, Brazil
gCorresponding Author: Raimundo Silva-Neto, Federal University of Delta of Parnaiba, Avenida Sao Sebastiao, 2819/Fatima, Parnaiba, PI 64001-020, Brazil
Manuscript submitted July 18, 2022, accepted October 27, 2022, published online November 10, 2022
Short title: Psychosis and BPFP Associated With COVID-19
doi: https://doi.org/10.14740/jmc3984
| Abstract | ▴Top |
Neuropsychiatric disorders associated with coronavirus infections emerged with the coronavirus disease 2019 (COVID-19) pandemic. We describe the clinical, laboratory and radiological features of a patient who presented, after recent COVID-19, two rare neuropsychiatric manifestations: a brief psychotic break followed by severe bilateral peripheral facial palsy.
Keywords: Complications of COVID-19; Neuropsychiatric disorders; Psychiatric disorder; Psychosis; Facial diplegia; Facial paralysis
| Introduction | ▴Top |
Coronavirus disease 2019 (COVID-19) has been associated with several central and peripheral psychiatric and neurological manifestations [1-3]. These manifestations can last for weeks or months, even after a mild infection. Some studies have investigated the long-term effects of the disease with an increasing number of cases of “long COVID-19” (symptoms beyond 3 weeks) and post-COVID-19 syndrome (beyond 12 weeks) [4]. Psychosis has been linked to pandemic respiratory viruses for several centuries [2, 5, 6]. The relationship between viral infections and peripheral facial paralysis is also well known [1, 7, 8].
The main psychiatric disorders resulting from COVID-19 are depression, post-traumatic stress disorder, obsessive compulsive disorder, and manic and psychotic symptoms [2, 3]. Some cases of acute and transient post-COVID-19 psychotic disorder have been reported since the beginning of the pandemic, despite being considered rare. It occurs most often in women in the second and third decades of life and lasts for less than 30 days. Remission is complete, and the patient returns to the premorbid state [3, 6]. To date, evidence on psychosis associated with recent COVID-19 or post-COVID-19 comes primarily from case reports [3, 9]. However, a recent population-based study showed an increased risk of new-onset psychosis in COVID-19 survivors, with an incidence of 0.4% at 6 months [3].
Among the neurological complications, the following were described: anosmia/ageusia, encephalitis, encephalopathy, ischemic stroke, intracranial hemorrhage, myelitis, Guillain-Barre syndrome, mild cognitive impairment, insomnia, headache and cranial nerve palsy [1, 9]. Bilateral peripheral facial palsy (BPFP) from post-COVID-19 is extremely rare, accounting for 0.3% of all peripheral facial palsies [1, 7-9]. In most cases, there are serious underlying medical conditions such as Guillain-Barre syndrome, multiple idiopathic cranial neuropathies, Lyme disease, acquired immunodeficiency syndrome, syphilis, sarcoidosis, tuberculosis, infectious mononucleosis, meningitis, leukemia, vasculitis, intrapontine or prepontine tumor or some rare syndromes (Melkersson-Rosenthal and Mobius). An extensive investigation of its etiology is required [7, 9-11]. Psychiatric disorders can both exacerbate and trigger neurological disorders and vice versa. Despite this, the two problems are not necessarily connected [1-4, 9, 12].
We report the case of a patient who presented with brief psychotic disorder and BPFP after COVID-19. These neuropsychiatric manifestations were thoroughly investigated and the definitive etiology was related to COVID-19. To the best of our knowledge, this is the first description of two rare pathologies in the same patient in a close temporal relationship with COVID-19. This report was authorized for publication by the patient who completed a signed informed consent form.
| Case Report | ▴Top |
Investigations
A 33-year-old male was diagnosed with COVID-19 on August 15, 2021. He had mild respiratory and systemic symptoms, not requiring hospitalization. On August 24, 2021, the patient no longer had any acute symptoms related to COVID-19. He was asymptomatic and tested negative for COVID-19 (RT-PCR test on nasal swab and oropharynx). He returned to his work and noted cognitive and executive difficulty. On August 26, 2021 (11 days after the diagnosis of COVID-19), during working hours, a psychotic break occurred. According to the patient: “It was frightening… I knew I was not normal, I noticed the acceleration of my thoughts, I had an unshakable conviction that I was very powerful, like a superhero, I felt superior to everyone”. On August 31, 2021, symptoms worsened. “I had a serious worry about sleeping, a huge and unshakable conviction that if I fell asleep, I would die. My wife was terrified and tried unsuccessfully to convince me otherwise, but I was relentless and very agitated. I was aware that my way of thinking was not normal”. On the day of the onset of psychiatric symptoms, the patient was hospitalized and evaluated by a psychiatrist.
On September 2, 2021, he already showed significant improvement in psychiatric symptoms. According to his wife, “he was already completely normal”. However, he started to present pain in the left retroauricular region. After 24 h (19th day after diagnosis of COVID-19), he reported paresis in the nasal and bilateral periorbital regions, with no movement of the forehead muscles, in addition to the impossibility of closing both eyes. Gradually, he could not move any muscles in his face. He consulted a neurologist who diagnosed severe BPFP, grade IV on the House-Brackmann scale [12] (Fig. 1). The remainder of the neurological examination revealed no abnormalities.
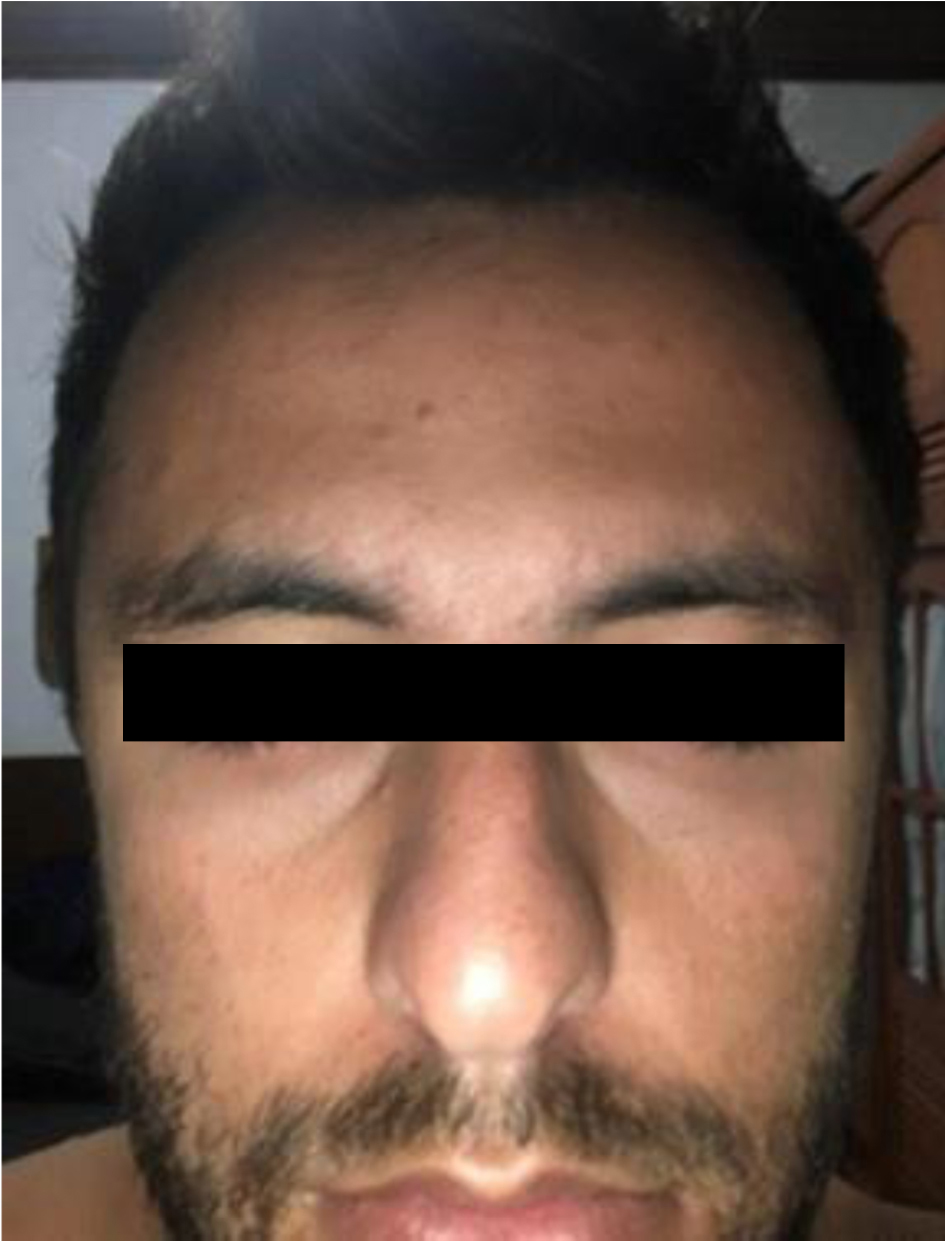 Click for large image | Figure 1. Bilateral peripheral facial palsy. |
Diagnosis
The patient had no comorbidity, no history of psychiatric illness or a previous report of psychosis. He did not use illegal drugs. There was no psychiatric illness among the first-degree relatives.
Blood tests (blood count, erythrocyte sedimentation rate (ESR), C-reactive protein, urea, creatine, thyroid hormones, electrolytes, glucose, glycated hemoglobin, liver tests, serum electrophoresis, antinuclear antibodies, serum angiotensin-converting enzyme, human immunodeficiency virus serology, Epstein-Barr virus and syphilis) did not reveal any abnormalities. Plain chest radiography was normal. Electroneuromyography showed markedly reduced contraction in bilateral facial muscles, but nerve conduction in the upper and lower limbs were normal, excluding Guillain-Barre syndrome. Brain magnetic resonance imaging (MRI) showed thickening and contrast enhancement of facial nerves, confirming bilateral facial neuritis (Fig. 2). Cerebrospinal fluid examination was normal.
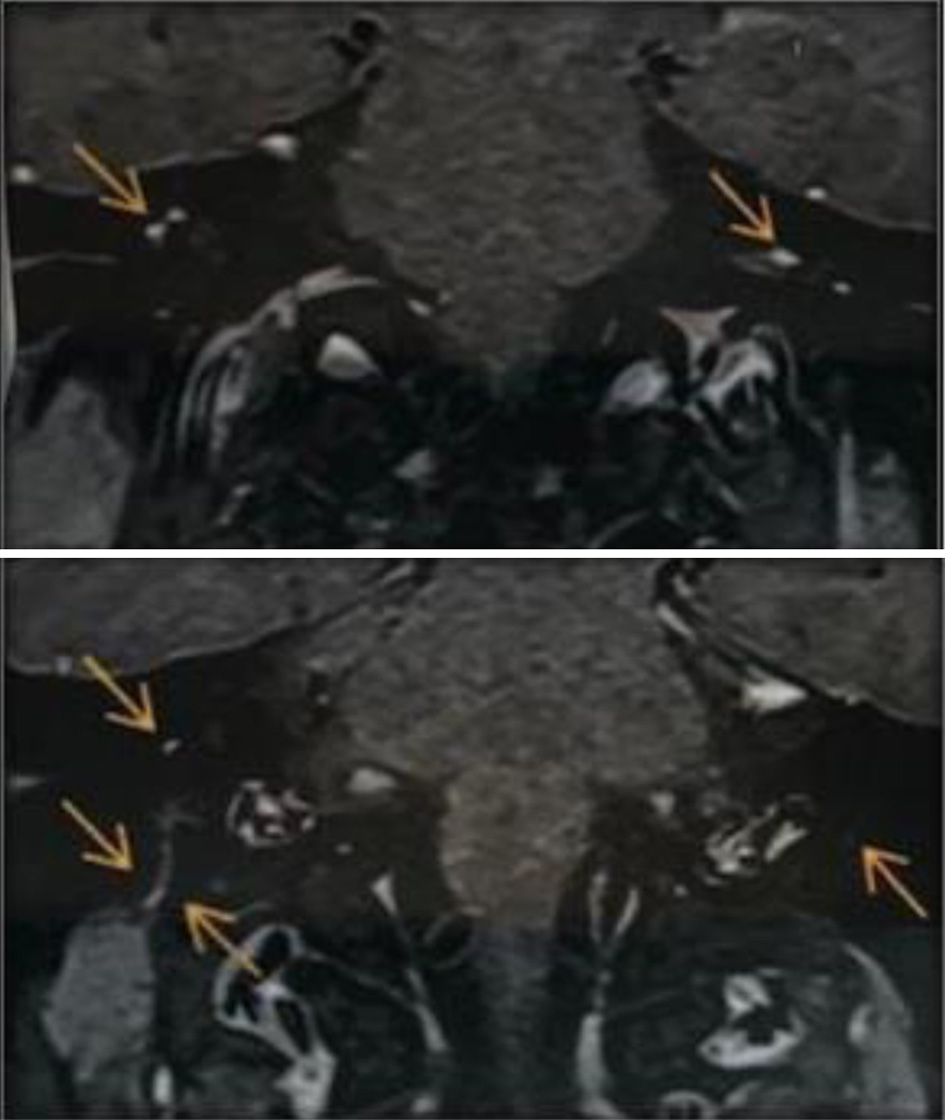 Click for large image | Figure 2. Magnetic resonance imaging of the brain and internal auditory canals. The arrows indicate thickening and enhancement of facial nerves. |
Treatment
On the day the psychiatric symptoms started, the patient was hospitalized and medicated with quetiapine 25 mg/day, escitalopram 10 mg/day, clonazepam 0.75 mg/day and lithium carbonate 450 mg/day. In the follow-up of BPFP, the patient was treated with acyclovir, prednisone and topical eye lubricant.
Follow-up and outcomes
The patient had a favorable evolution, with partial recovery in 58 days and full recovery of BPFP at 77 days. At 9 months of follow-up, the patient no longer had psychotic episodes or repeated facial paralysis.
| Discussion | ▴Top |
This case report provides relevant clinical details that are not present in population studies, clearly ruling out the risk of bias in the diagnosis of psychosis associated with recent COVID-19, namely, 1) other causes of psychosis were ruled out, in addition, a psychiatrist made a detailed evaluation for delirium; 2) with the evolution of neurological complications, neuroimaging exams, complete cerebrospinal fluid (CSF) study, blood tests and electroneuromyography were complemented; 3) the close temporal relationship of neuropsychiatric signs or symptoms and their resolutions ruled out indirect effects on people’s mental health, such as those mediated by physical distancing measures, such as self-isolation or quarantine; 4) other comorbidities were ruled out by clinical, neurological and psychiatric examinations.
In this report, the patient had a brief psychotic break lasting 7 days, 11 days after the onset of COVID-19 symptoms. The psychiatric symptoms reported by the patient can be characterized as paranoid and self-referential delusions with confusional state. Usually, these symptoms occur at least 2 weeks after the first somatic manifestations attributed to COVID-19, with a short duration ranging from 2 to 90 days [2, 13].
This psychotic break showed typical features of psychotic episodes secondary to recent viral infection, such as: 1) absence of predisposing factors, such as a family history of serious mental illness or substance abuse; 2) atypical age of onset; 3) subacute onset of psychotic symptoms (less than 1 week) and rapid recovery (maximum of 2 weeks) with low doses of antipsychotics; and 4) presence of confusion mixed with typical psychotic symptoms [13].
Healthcare professionals need to be aware of the existence of psychotic disorder after COVID-19 infection and the likelihood of a good prognosis. We have seen rapid recovery with low-dose antipsychotics, similar to other psychoses caused by viral infections that have been described in patients with COVID-19 [2, 13].
After remission of psychosis, the patient developed BPFP, a rare neurological manifestation. Therefore, he underwent numerous diagnostic tests to exclude other secondary causes. The importance of considering the numerous diseases that present with BPFP is to exclude fatal or life-threatening diseases [10].
All differential diagnoses were extensively investigated. Guillain-Barre syndrome, an inflammatory post-infectious polyradiculoneuritis, can arise after infections by viruses, including the coronavirus. There are reports of BPFP associated with post-COVID-19 Guillain-Barre syndrome [9-11]. However, this syndrome was easily excluded by the absence of strength deficit and profound areflexia and by the CSF and electroneuromyographic examinations.
Cerebellopontine angle tumors were excluded by brain MRI. Chest radiography and angiotensin-converting enzyme dosage excluded the diagnosis of sarcoidosis. Studies have shown that diabetic patients are more prone to facial nerve degeneration and BPFP may be present in up to 28% of cases [9, 10]. The patient’s blood glucose was normal.
There are three explanations for the emergence of neuropsychiatric manifestations in patients with COVID-19: 1) direct effect of the virus on the central nervous system; 2) indirect effect (inflammatory reactions mediated by cytokine storm, metabolic disturbances and hypoxia from severe pneumonia); and 3) adverse effects of pharmacological treatments used against the virus, including corticosteroids [1, 2, 13, 14].
In our case, IgG, IgM, and C-reactive protein for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in CSF were negative, similar to the cases previously described [1, 8-10]. Based on these findings, we hypothesized that the patient’s manifestations were due to cytokine storm-mediated immune-mediated inflammatory reactions rather than direct viral action [9].
We believe that our patient had BPFP as a result of COVID-19, as there was a close relationship between this infection and facial nerve palsy and other secondary causes were excluded. In addition to SARS-CoV-2 infection, paralysis of this nerve is reported to be associated with anti-COVID-19 vaccination, although the pathophysiological mechanism remains unclear [15, 16]. We hypothesized that the possible etiopathogenesis of facial paralysis in COVID-19 and post-vaccination patients is similar. Peripheral facial palsy is related to immunological mechanisms and inflammatory reactions mediated by cytokine storms rather than direct viral action [14].
We found only three cases of SARS-CoV-2-related psychiatric symptoms that were clearly linked to hypoxic or encephalitic brain injury. This finding is consistent with the rarity of case reports that have associated central nervous system (CNS) detection of coronavirus with acute encephalitis or encephalomyelitis, particularly in immunocompromised children [17].
When evaluating a psychotic patient, it is necessary to exclude clinical situations, such as infectious diseases (infectious mononucleosis, human immunodeficiency virus (HIV), syphilis), inflammatory diseases (multiple sclerosis, sarcoidosis, Guillain-Barre syndrome) and metabolic causes (diabetes mellitus). Our patient was evaluated by several doctors (clinicians, psychiatrist and neurologist) and performed several complementary exams. Therefore, the diagnosis of psychotic disorder due to a general medical condition and drug-induced psychotic disorder were evidently ruled out.
Researchers evaluated, through MRI, a group of post-COVID-19 patients and demonstrated a reduction in the overall size of the brain when compared to the non-COVID-19 group. They found altered connections between different brain regions, especially in the olfactory cortex. Patients in the post-COVID-19 group also performed worse on a test of attention and mental flexibility, a finding that was associated with volume reductions in a part of the cerebellum related to smell and social relationships. New neuroimaging evidence suggests that areas of the brain may undergo neuroplasticity and shrink in size in both mild COVID-19 and first-episode psychosis. These areas are the orbitofrontal cortex and the parahippocampal gyrus [18].
Patients with brief psychotic disorder generally have a good prognosis and most do not have other major psychiatric problems. The duration of acute and residual symptoms is often only a few days. Sometimes, depressive symptoms arise after the resolution of psychotic symptoms and suicide is a concern. Patients with acute psychosis may require brief hospitalization for both evaluation and protection. Our patient had a good clinical evolution with complete recovery of psychiatric symptoms in a short period of time and use of a low-dose antipsychotic medication.
The mainstays for the treatment of peripheral facial palsy are supportive care and oral steroids [1]. The prognosis is good in most cases [1, 8, 9]. Our patient had full recovery of PFPB in 77 days.
Conclusions
BPFP and brief psychotic disorder are rare neuropsychiatric manifestations and may be complications of COVID-19, suggesting an indirect immune-mediated mechanism rather than direct virus-induced damage.
Learning points
COVID-19 has several neurological and psychiatric complications and some may be rare, like those of our patient. It appears that these complications are self-limiting.
Acknowledgments
None to declare.
Financial Disclosure
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article
Informed Consent
Informed consent was obtained.
Author Contributions
All the authors contributed to the article conception, design, and data collection. The first draft of the manuscript was written by CJ, and all authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Lima MA, Silva MTT, Soares CN, Coutinho R, Oliveira HS, Afonso L, Espindola O, et al. Peripheral facial nerve palsy associated with COVID-19. J Neurovirol. 2020;26(6):941-944.
doi pubmed - Smith CM, Gilbert EB, Riordan PA, Helmke N, von Isenburg M, Kincaid BR, Shirey KG. COVID-19-associated psychosis: A systematic review of case reports. Gen Hosp Psychiatry. 2021;73:84-100.
doi pubmed - Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416-427.
doi - Halpin S, O'Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242-1243.
doi pubmed - Kulaga SS, Miller CWT. Viral respiratory infections and psychosis: A review of the literature and the implications of COVID-19. Neurosci Biobehav Rev. 2021;127:520-530.
doi pubmed - Smith CM, Komisar JR, Mourad A, Kincaid BR. COVID-19-associated brief psychotic disorder. BMJ Case Rep. 2020;13(8):e236940.
doi pubmed - Kerstens J, Deschuytere L, Schotsmans K, Marechal E. Bilateral peripheral facial palsy following asymptomatic COVID-19 infection: a case report. Acta Neurol Belg. 2021;121(3):815-816.
doi pubmed - Cabrera Muras A, Carmona-Abellan MM, Collia Fernandez A, Uterga Valiente JM, Anton Mendez L, Garcia-Monco JC. Bilateral facial nerve palsy associated with COVID-19 and Epstein-Barr virus co-infection. Eur J Neurol. 2021;28(1):358-360.
doi pubmed - Goh Y, Beh DLL, Makmur A, Somani J, Chan ACY. Pearls & Oy-sters: Facial nerve palsy in COVID-19 infection. Neurology. 2020;95(8):364-367.
doi pubmed - Pothiawala S, Lateef F. Bilateral facial nerve palsy: a diagnostic dilemma. Case Rep Emerg Med. 2012;2012:458371.
doi pubmed - Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574-2576.
doi pubmed - House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147.
doi pubmed - Parra A, Juanes A, Losada CP, Alvarez-Sesmero S, Santana VD, Marti I, Urricelqui J, et al. Psychotic symptoms in COVID-19 patients. A retrospective descriptive study. Psychiatry Res. 2020;291:113254.
doi pubmed - da Silva Lopes L, Silva RO, de Sousa Lima G, de Araujo Costa AC, Barros DF, Silva-Neto RP. Is there a common pathophysiological mechanism between COVID-19 and depression? Acta Neurol Belg. 2021;121(5):1117-1122.
doi pubmed - Cellina M, D'Arrigo A, Floridi C, Oliva G, Carrafiello G. Left Bell's palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: A case report. Clin Imaging. 2022;82:1-4.
doi pubmed - Khurshid M, Ansari I, Ahmad H, Ghaffar H, Khurshid A, Shahid A, Essar MY, et al. Development of facial palsy following COVID-19 vaccination: A systematic review. Ann Med Surg (Lond). 2022;82:104758.
doi pubmed - Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
doi - Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268-2270.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


