| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 11, November 2022, pages 561-568
Extramedullary Myeloid Leukemia in the Setting of a Myeloproliferative Neoplasm
Jorgena Kostia, c, Timothy Mervakb, Howard Terebeloa
aDepartment of Hematology and Oncology, Ascension Providence Hospital, Southfield, Michigan and Michigan State University, East Lansing, MI, USA
bDepartment of Pathology, Ascension Providence Hospital, Southfield, MI, USA
cCorresponding Author: Jorgena Kosti, Department of Hematology and Oncology, Michigan State University, Ascension Providence, 22301 Foster Winter Drive, Southfield, Michigan, 48075, USA
Manuscript submitted August 15, 2022, accepted November 4, 2022, published online November 27, 2022
Short title: EML in the Setting of MPN
doi: https://doi.org/10.14740/jmc3996
| Abstract | ▴Top |
Extramedullary acute myeloid leukemia (EML), also known as myeloid sarcoma (MS), is an extramedullary solid mass derived from the proliferation of myeloblasts outside of the bone marrow. EML can present independently or concurrently with intramedullary acute myeloid leukemia (iAML). It can happen de novo or secondary to iAML, myeloproliferative neoplasm (MPN), chronic myelomonocytic leukemia (CMML), or myelodysplastic syndrome (MDS). We present a 57-year-old female with a history of Janus kinase 2 (JAK-2)-positive essential thrombocythemia (ET) evolving into EML in the setting of a persistent TP53 mutation. We discuss the essential diagnostic studies including tissue biopsy and fluorodeoxyglucose positron emission tomography/computed tomography (F-FDG PET/CT) imaging. We also investigate the significance of cytogenetics and next-generation sequencing (NGS) along with the unique pathogenesis, treatment and prognostic implications.
Keywords: Myeloid sarcoma; JAK-2-positive essential thrombocythemia; TP53; Venetoclax; 5- azacitidine; Eprenetapopt; CD33 CAR T-cell therapy
| Introduction | ▴Top |
Extramedullary acute myeloid leukemia (EML) is a solid constituent formed by the migration and proliferation of myeloblasts outside of the intramedullary tissue [1]. Even though its incidence is not well reported [2], EML is estimated to occur in about 2-8% of the adult population with intramedullary acute myeloid leukemia (iAML) [3]. Myeloid sarcoma (MS) most commonly presents with concomitant iAML, but it can also present as a recurrence of it or de novo [2]. EML can also occur secondary to other hematological conditions such as myeloproliferative neoplasm (MPN), myelodysplastic syndrome (MDS), and chronic myelomonocytic leukemia (CMML) [1]. EML has a myriad of presentations, most commonly found in bone, lymphoid tissue, gastric mucosa, breast, skin, and in rare cases in the central nervous system (CNS) [4-6]. In the pediatric population, leukemia cutis is the most common presentation [7, 8]. MS resembles solid cancers in histology, immunohistochemistry (IHC), and RNA sequencing [9]. Its morphology can be described as a single-filing architectural pattern, identical to that of invasive lobular breast carcinoma and with dense desmoplastic keloid-like fibrosis, similar to colon, gallbladder, and pancreatic carcinomas [9].
Cytogenetic and molecular studies have identified similar mutation patterns as those found in AML [10, 11]. Prognostic cytogenetic and molecular abnormalities have not been determined yet in EML; however, reported cases show that the presence of MS confers a worse prognosis [12]. In recent clinical practice, the treatment modalities for EML incorporate surgery and radiation therapy that can be utilized in localized disease as well as in the palliative setting [13]. The definitive treatment approach almost always requires the addition of systemic therapy usually in the form of AML induction chemotherapy, as well as consolidation with allogeneic stem cell transplantation (allo-SCT) in the first line setting [13]. New targeted therapies and chimeric antigen receptor (CAR) T-cell therapy comprise a potential therapeutic breakthrough for the future. In this paper, we present a case of extramedullary bone and epidural involvement of AML arising from an underlying MPN.
| Case Report | ▴Top |
Investigations
We present a case of a 57-year-old female with history of cerebral aneurysm, transient ischemic attack and JAK-2-positive essential thrombocythemia (ET). The patient was initially diagnosed in 1987 and progressed to spent-phase myelofibrosis in 2011. The bone marrow pathology at the time of spent phase diagnosis revealed myelofibrosis with no increase in blast count and a decision was made to treat the patient with hydroxyurea. Six years later, she presented to the hospital with debilitating low back pain and pancytopenia.
Diagnosis
On presentation, a magnetic resonance imaging (MRI) with contrast of the lumbar spine showed multiple lesions throughout the lumbar spine and a mass centered at the S1 vertebrae extending into the epidural space as well as into the S1-S2 neural foramen (Fig. 1). The surgical sacral mass biopsy was indicative of a small blue cell neoplasm favoring MS. The tissue stained positive for CD34, CD117 (not shown), CD99 (not shown), CD33, CD11c, and equivocal for CD 68-KPI (Fig. 2). Negative stains included CD43 (weak), CD45, CD56, CD13, TdT, and myeloperoxidase (MPO). The bone marrow biopsy indicated presence of AML, arising within a chronic MPN (Fig. 3). Approximately a third of the marrow space was involved by a dense proliferation of primitive cells. The remainder of the marrow demonstrated marked megakaryocytic expansion with abnormal forms associated with scattered erythroid and myeloid precursors. The reticulin stain revealed marked reticulin fibrosis in much of the marrow. The bone marrow aspirate was insufficient for evaluation. Cytogenetic studies revealed a normal karyotype. The fluorescence in situ hybridization (FISH) study was normal and the next-generation sequencing (NGS) panel identified a TP53 Arg273His mutation with 17% allele frequency.
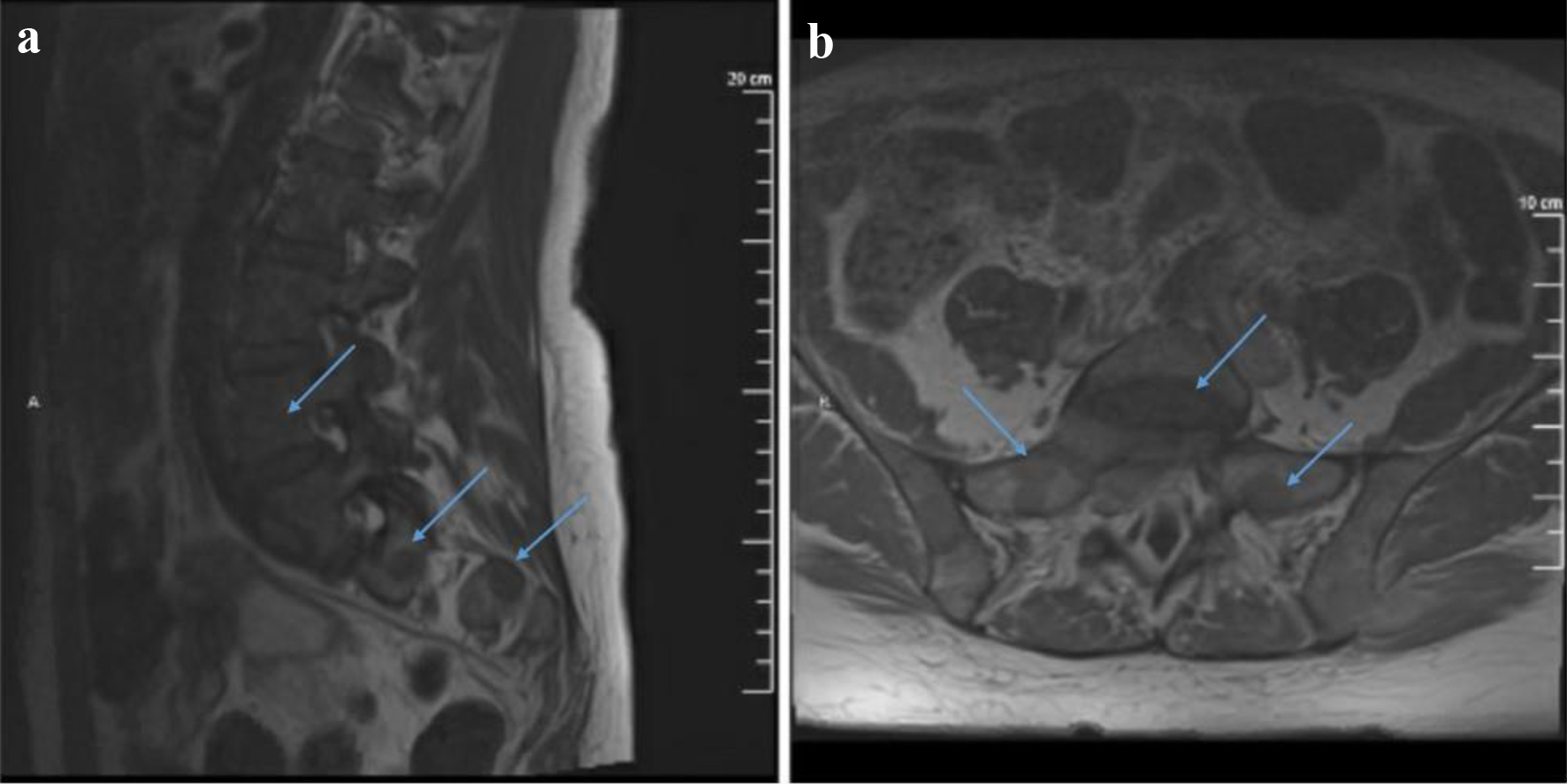 Click for large image | Figure 1. T1-weighted MRI with contrast sagittal (a) and axial (b) views showing multiple lesions throughout the visualized spine. A mass centered at S1 extends into the epidural space as well as into the right S1-S2 neural foramen and compresses upon the exiting S1 nerve root. It also shows possible involvement of the right S2 neural foramen by an additional lesion. MRI: magnetic resonance imaging. |
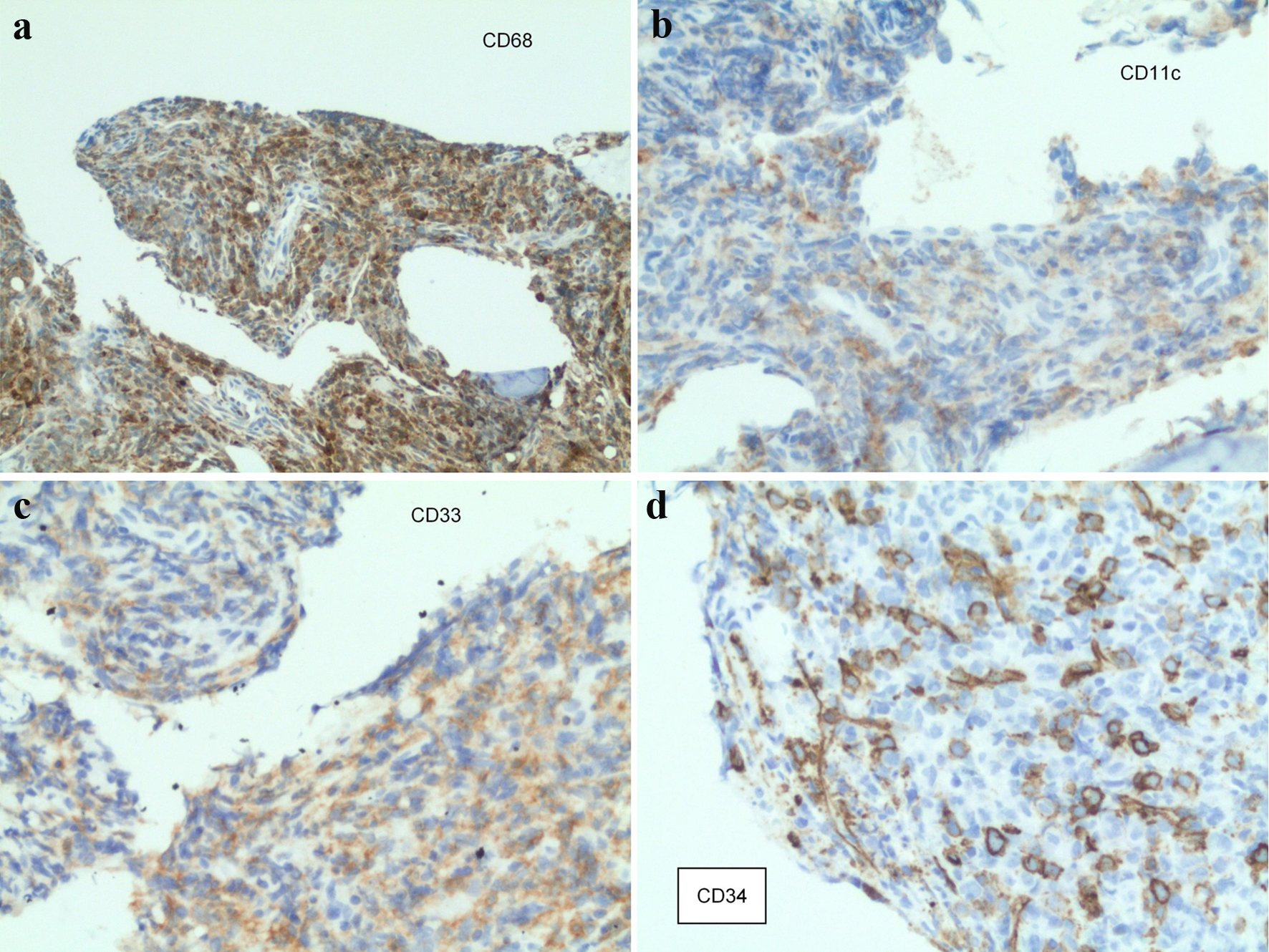 Click for large image | Figure 2. Staining from the sacral mass biopsy: (a) CD68-positive cells; (b) CD11c-positive cells; (c) CD33-positive cells; (d) CD34-positive cells. |
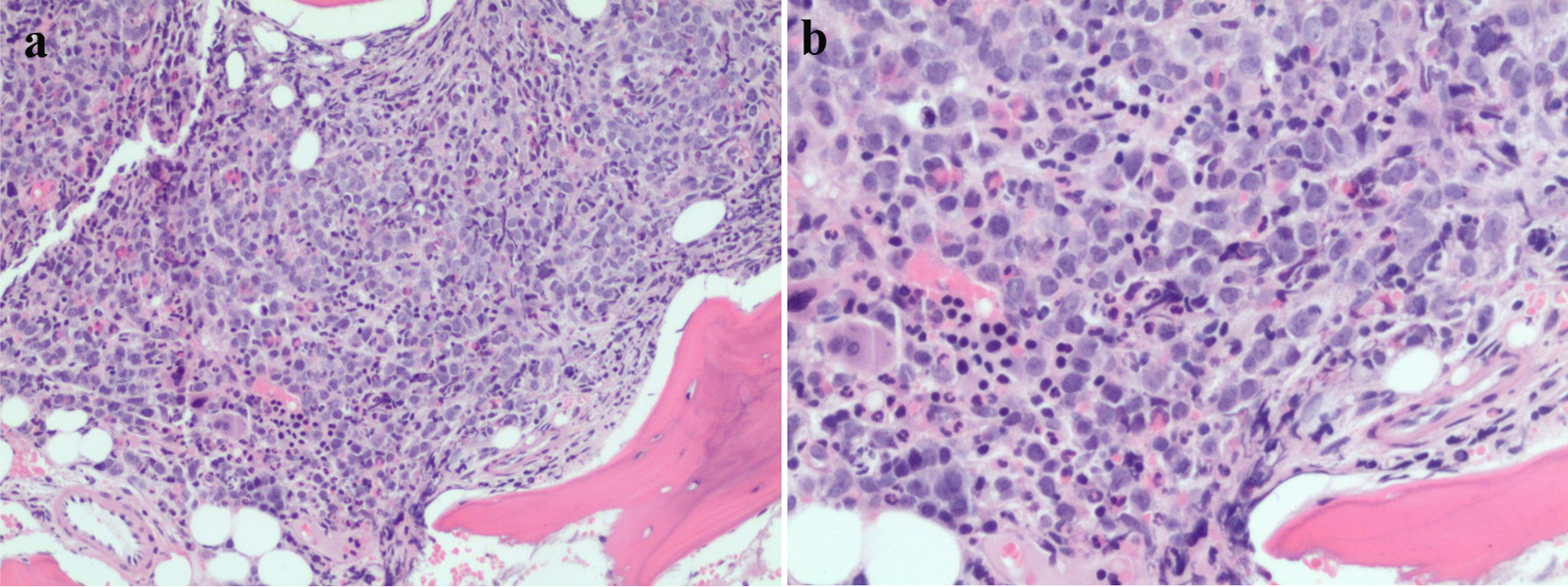 Click for large image | Figure 3. (a, b) Bone marrow pathology sample showing increasing mononuclear cells. |
Treatment
The patient underwent a decompressive laminectomy for a right L5-S1 epidural tumor. Next, she received one fraction of radiation therapy to the S1 joint which resulted in the improvement of her lower back pain. She was subsequently induced with cytarabine and daunorubicin 7 + 3 protocol.
Follow-up and outcomes
A repeat bone marrow biopsy at day 14 after induction chemotherapy revealed a hypocellular marrow with less than 5% cellularity. The stain for CD34 was largely negative. There were mostly areas of dense fibrosis with few lymphoid cells coinciding with myelofibrosis. There was no evidence of AML on flow cytometric evaluation. The bone marrow aspirate was insufficient for evaluation. A bone marrow biopsy on day 28 was positive for an MPN (post-ET myelofibrosis). There was no morphological or immunophenotypic evidence of AML. The marrow had a cellularity of 95-100% with 1% blasts present. The repeat NGS panel identified a persistent TP53 Arg273His mutation with unchanged allele frequency at 17%.
Two months after, a repeat MRI with contrast of the lumbar spine redemonstrated marrow signal abnormality identified throughout the lumbosacral spine concerning for interval progression of prior EML. The epidural mass described previously, at the level of S1, appeared less prominent (Fig. 4). A positron emission tomography/computed tomography (PET/CT) identified focal areas of fluorodeoxyglucose (FDG) activity within the posterior left iliac bone as well as in the sacral region (Fig. 5). A bone biopsy of the left iliac bone showed bone remodeling fibrotic changes. A repeat biopsy of the prior soft tissue area near the right sacroiliac (SI) joint showed fatty tissue with necrosis. Based on the biopsy results, we concluded that the patient had regenerative bone changes as opposed to relapsed leukemia. A repeat bone marrow biopsy showed stromal changes including loose fibrosis and maturing trilineage hematopoiesis. The marrow had 30-45% cellullarity and 1% blasts. The flow cytometry was negative. The bone marrow biopsy was compatible with an underlying MPN, and showed no evidence of AML. The TP53 Arg273His mutation persisted in the bone marrow with 25% allele frequency, which had increased since the prior biopsy. The patient received four cycles of 5-azacytidine and venetoclax with goals to bridge to allo-SCT and suppress the TP53 mutation. She received full intensity fludarabine and busulfan and then proceeded with allo-SCT from a 10/10 matched unrelated donor. A repeat bone marrow biopsy 60 days after transplant did not show any evidence of iAML. The cytogenetics and FISH analysis were normal, but the TP53 mutation testing was not done at the time. Shortly after, the patient was admitted with altered mental status and was found to have leukemic meningitis. At this point, the patient decided to pursue hospice care. The time from her diagnosis of EML to her death was 9 months.
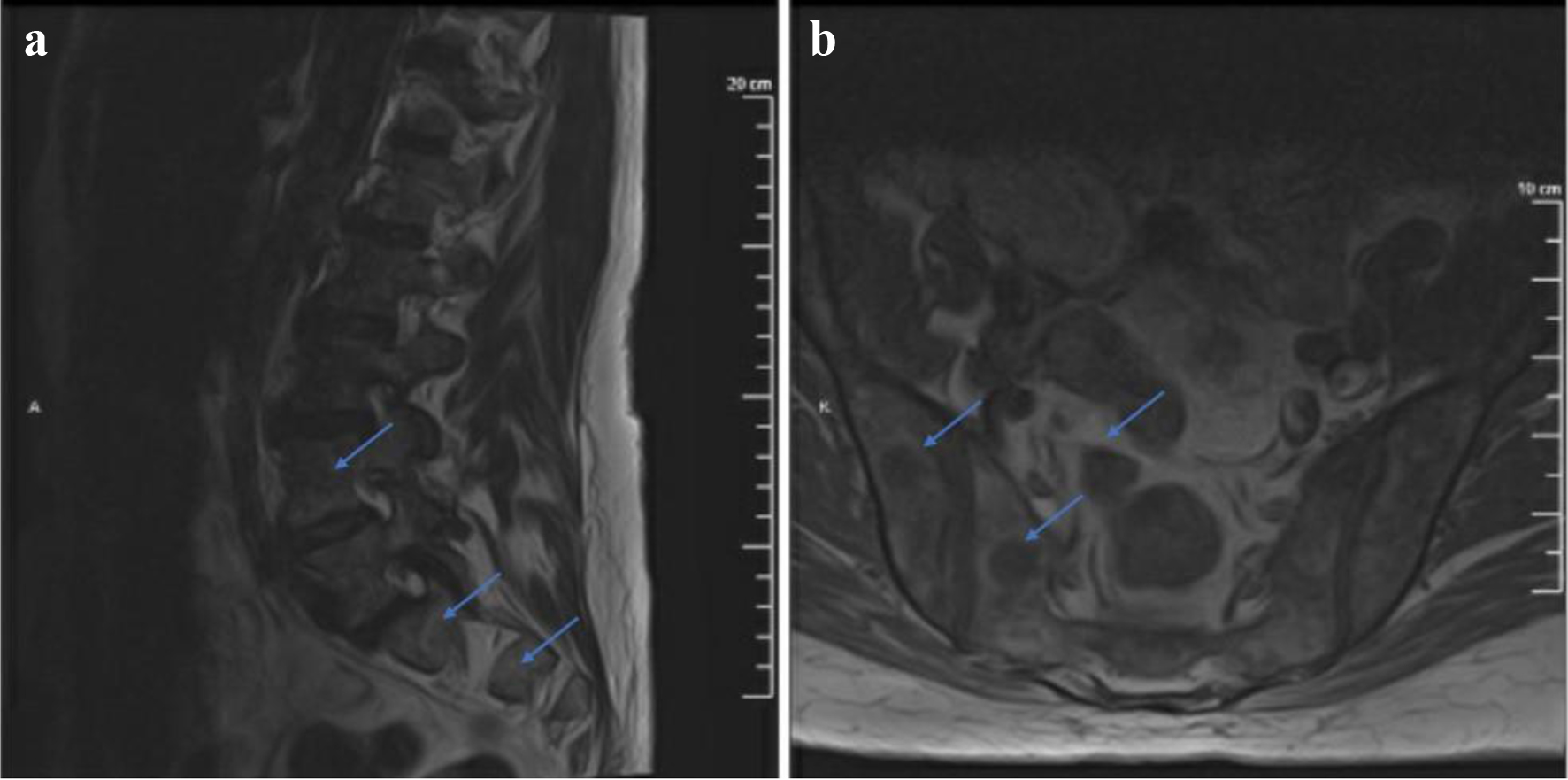 Click for large image | Figure 4. T1-weighted MRI with contrast sagittal (a) and axial (b) views showing marrow signal abnormality identified throughout the lumbosacral spine. The S1 mass described previously appears less prominent. MRI: magnetic resonance imaging. |
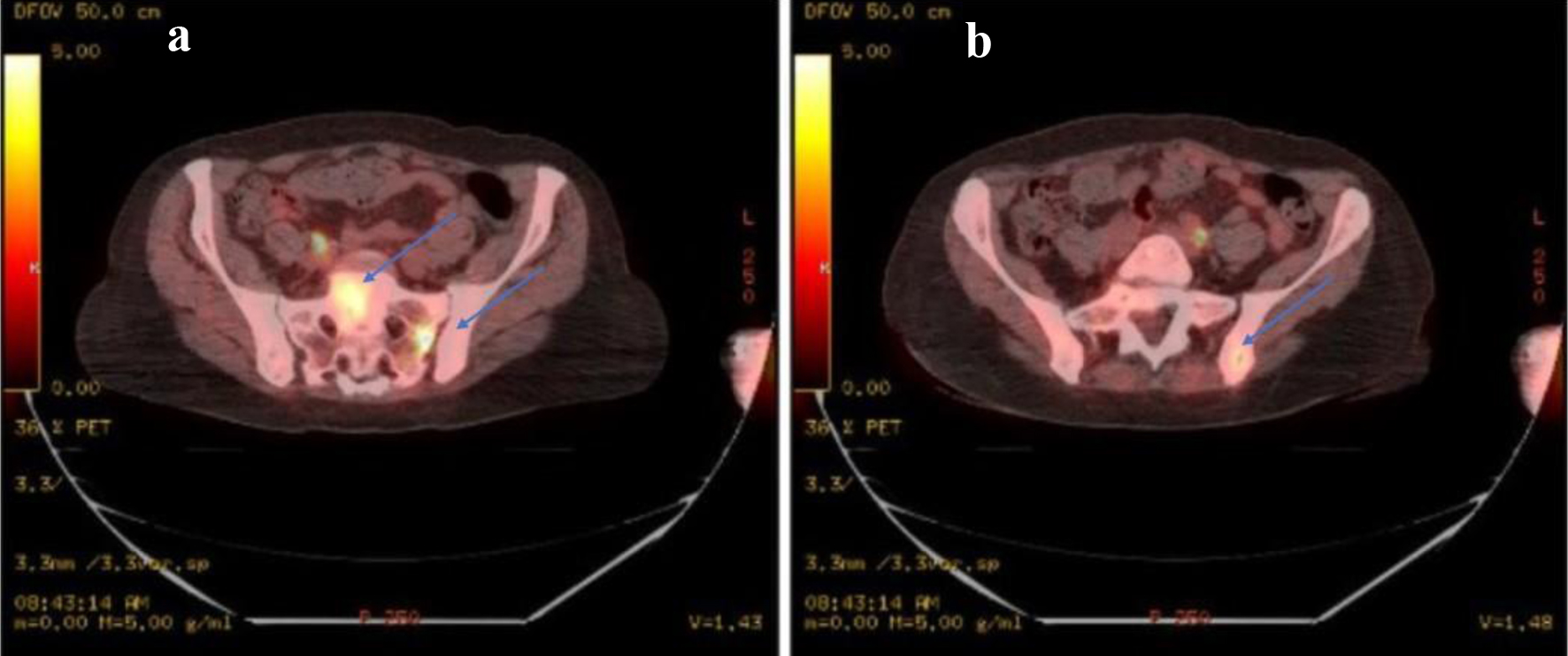 Click for large image | Figure 5. (a) PET-CT identifying focal areas of SUV activity. There are intense areas of activity identified within the sacrum, in the left sacral alar with an average SUV of 1.9 and a maximum SUV of 4.3 as well as in the mid sacrum S1 level with an average SUV of 2.1 and a maximum SUV of 4.2. (b) There is an average SUV of 1.6 with maximum of 3.0 in the posterior left iliac bone adjacent to the sacroiliac joint as well. Discrete lesions in these areas are not identified. PET: positron emission tomography; CT: computed tomography; SUV: standard uptake value. |
| Discussion | ▴Top |
The diagnosis of MS involves a multi-modality approach. Imaging is one of the first diagnostic tools that can detect a suspicious mass early and it can also be used to evaluate treatment response [13, 14]. PET/CT scans should be considered in diagnosing new-onset EML, as well as relapsed EML, because it can help detect small lesions that are difficult to visualize with other imaging modalities [15]. PET/CT scans aid in facilitating diagnosis as well as identifying obscure foci of relapsed disease [16]. A retrospective analysis showed that an F-FDG-PET/CT is more accurate than an F-FDG-PET or CT alone [17]; however, in both cases, bone marrow and tissue biopsies are essential for diagnosis [13]. The differential of EML includes Ewing’s sarcoma, peripheral neuroendocrine tumors, neuroblastomas, lymphoblastic lymphomas, Wilms’ tumors, sarcomas, and Burkitt’s lymphoma. Often MS resembles small, round, blue-cell tumors that are embryonic in appearance [18, 19]. Morphologically, EML is characterized by infiltrative myeloblasts derived from adnexal destruction [20]. In regards to IHC, CD68-KP1 is the most expressed marker followed by MPO [13, 20, 21]. Other markers include CD34, CD43, CD45, CD68, CD56, CD117, CD11c, CD13, and CD33 [13, 19, 20]. Cytogenetic aberrations correlate closely with those found in AML, MPN, or MDS. The most common cytogenetic abnormalities are t(8;21)(q22;q22) and inv(16)(p13;q22) which are also associated with AML subclassifications with the most favorable prognosis [20, 22-24]. Even though cytogenetics can characterize MS, their prognostic value is uncertain in EML. In our patient’s case, the NGS panel only revealed a TP53 mutation. In other reports, FLT3 and NPMI mutations have shown to be the most common mutations found in EML case reports [20, 22-24]. Gene encoding tyrosine kinases (FLT3, KIT, and KRAS), tumor suppressors genes (WTI and TP53), spliceosome proteins (SF3B1 and SRSF2), transcription factors (RUNX1), as well as epigenetic modifiers (TET2 and ASXL1) are commonly found in EML [11, 24].
The incidence of the TP53 mutation is only 12.7% in AML; however, it reaches an incidence up to 40-50% in solid malignancies [25-27]. In AML, the TP53 mutation is most commonly associated with the use of prior chemotherapy or with the presence of a complex karyotype. TP53 mutations have also been found in secondary AML associated with polycythemia vera (PV) and ET [25, 28]. We identified five cases of MS associated with TP53 mutations published in literature [29]. One of these cases reported a mediastinal MS preceding AML with a PICALM-MLLT10 fusion gene mutation in addition to the TP53 mutation [29]. Two other cases reported de novo MS and two additional cases had underlying MDS before developing MS [1, 11]. Pre-clinical studies have shown that inactivation of TP53 leads to uncontrolled cell proliferation and increased oncogenesis in hematopoietic stem cells as well as enhanced engraftment in various tissues [27]. In mice, TP53-deficient cells were shown to have an engraftment advantage over the TP53 wild-type stem cells which could potentially aid in the engraftment of myeloblasts to the extramedullary tissues [30-34]. This could relate to our patient’s case, where a TP53 mutation may have helped the development of MS. TP53 is often seen as a later finding in hematological malignancies, as it is known to confer poor prognosis.
The pathogenesis of EML has yet to be fully elucidated. For a long time, CD56, the blast neural cell adhesion molecule, was a protein of high interest in the mechanism of homing of myeloid blasts to various extramedullary sites that expressed high CD56 [10, 20, 35]. High CD56 expression is most commonly associated with t(8;21) and 11q23 aberrations [35, 36]; however, most patients with MS do not express CD 56 in their leukemic cells [12, 37, 38]. Another important cell adhesion protein is CD11b (surface β2-integrin member macrophage-1 antigen) which is derived from mononuclear cells and is found to be highly expressed in AML with monoblastic/myelomonocytic differentiation, where the risk of developing MS is higher [38, 39]. Studies have shown the expression of CD11b in AML associated with MS to be higher than in AML alone [37]. The RAS-MAPK/ERK pathway is another potential pathway in the development of EML as metastasis-suppressor RAF kinase inhibitor protein (RKIP) loss has been reported in 50% of patients with AML associated with MS, versus only 14% in patients with AML alone [40].
Several factors need to be taken into consideration while choosing therapy for the management of MS, such as the timing of initial diagnosis versus relapse, the presence of concurrent AML, and the presence of local symptoms [20]. Local therapies such as surgical decompression or radiation therapy have been used in isolated MS cases, as well as in cases of severe symptoms with palliative intent [1]. In our patient with bone involvement, surgical decompression followed by radiation therapy played a major role in her treatment approach, as well as in the relief of her symptoms. The risk for impending cord compression remains a real risk for patients with epidural/bone involvement. Even though our patient did not have cord compression, her symptoms required surgical intervention. Systemic therapy, however, should almost always be considered in both local and metastatic disease. Clinical data have shown that 75-90% of patients with isolated MS who do not receive systemic therapy progress to AML within 4 months [12, 41].
AML induction chemotherapy has found usefulness in the treatment of EML since this is preceded or followed by iAML within a short period of time in most cases [42]. The current suggested approach is utilizing anthracycline-based induction regimens, and allogeneic transplantation in the initial setting [13, 43]. The presence of EML itself confers a poor prognosis in patients with iAML [12]. A series of case reports have demonstrated that addition of systemic therapy to surgery and radiation can achieve a 65% remission rate and an overall survival (OS) of 40 months in patients with EML [13, 44]. Retrospective data suggest that allo-SCT should be offered in the first line setting, after induction chemotherapy in patients with isolated EML as well as in patients with concurrent iAML [13, 43, 45]. Patients who benefit the most from allogeneic transplant are those who achieve complete response (CR) prior to it and second transplants are sometimes indicated as salvage therapy in patients with relapsed disease [12, 46-49].
While induction chemotherapy with cytarabine and anthracycline combination is the preferred initial treatment in fit AML patients, this is not the case in TP53-mutated patients due to the discouraging data in this group [25]. More recently, the combination of venetoclax and the hypomethylating agent (HMA) 5-azacytidine has gained great interest in the treatment of TP53-mutated patients. In a recent randomized prospective trial of older AML patients, the doublet was associated with a higher incidence of composite remission in the TP53-mutated group, but this was not translated into OS benefit [50]. The mechanism of apoptosis mediated by venetoclax appears to be TP53-independent, while HMA agents have shown up to 47% response rates in TP53-mutated patients [51, 52]. Currently, there are several clinical trials investigating novel therapies in this group of patients. The mutant p53 reactivator, eprenetapopt (APR-246), has shown some promise. Phase I/II data show the combination of APR-246 with 5-azacitidine in patients with TP53-mutant MDS and AML has achieved a CR of 44% [53]. TP53 mutation predicted higher CR when this regimen was given as maintenance therapy after allo-SCT [52]. An anti-CD47 IgG4 monoclonal antibody that stimulates cellular phagocytosis and T cell-mediated cytotoxic effect on leukemic cells called magrolimab is also being investigated in combination with azacytidine in the untreated AML as well as relapsed setting. In the TP53-mutant cohort, this combination has shown a CR of 45% with a median duration of response of 7.6 months [52]. Other targeted therapies that are being investigated are eprenetapopt in combination with venetoclax and azacytidine and bispecific dual affinity retargeting antibody (DART) against CD3 and CD123, flotetuzumab in the early relapse setting [52].
Non-randomized data that support venetoclax combination with HMA in AML patients also exist in the salvage setting. This is supported by a small retrospective analysis of AML patients that reported a CR of 58% with venetoclax and 5-azacytidine in the salvage/pre-transplant setting [54]. We thought that this combination of therapy would have benefited our patient as a bridge to allo-SCT, by hopefully inducing CR. In the relapse setting, other potential targeted therapies that have shown activity in relapse EML based on reported patient cases are anti-CD33 monoclonal antibody gemtuzumab ozogamicin in patients with concurrent positive CD33 AML [55] and the tyrosine kinase inhibitor imatinib for patients with FIP1L1-PDGFRA and BCR-ABL mutations [13, 56]. The newest consideration for relapse EML is the PRGN-3006 ultra-CAR-expressing T-cell therapy, which is currently under investigation in a phase 1/1b trial. This is a multigenic, autologous CD33 CAR T-cell therapy trial that is currently open for enrollment for relapsed/refractory AML, including extramedullary disease [57].
Learning points
MSs are infrequent and vexing for the clinician when it comes to making a diagnosis and approaching treatment outcomes.
We hypothesize that TP53 mutation may have been the initiating event for developing EML and its persistency may have been a major factor of contribution in this patient’s poor prognosis.
Localized treatment with surgery and radiation in conjuncture to AML-based systemic therapy and stem cell transplantation remain the current approach to treating EML, although the outcomes are poor overall.
TP53-mutated AML patients have poor outcomes with standard therapy, therefore targeted therapies and clinical trials should be considered. We can predict similar poor outcomes in EML with TP53 mutations.
Considerations for targeted molecular therapies in relapse MS are anti-CD33 monoclonal antibody gemtuzumab ozogamicin, as well as the tyrosine kinase imatinib.
CD33 CART therapy is currently under investigation for relapse disease, including EML, in the clinical trial setting.
Further large randomized controlled studies are necessary to establish these and other novel agents as the standard of care for the treatment of EML.
In general, extramedullary presentations are unique and not fully understood. Finding the best treatment approach poses a complicated challenge.
Acknowledgments
None to declare.
Financial Disclosure
We did not require any funding.
Conflict of Interest
We do not have any conflict of interest.
Informed Consent
Verbal consent from the patient’s family was obtained.
Author Contributions
Dr. Jorgena Kosti gathered the patient’s information and wrote the manuscript. Dr. Timothy Mervak provided the pathology and the pathology slides as well as pictures. Dr. Howard Terebelo edited and supervised the writing of this paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kaur V, Swami A, Alapat D, Abdallah AO, Motwani P, Hutchins LF, Jethava Y. Clinical characteristics, molecular profile and outcomes of myeloid sarcoma: a single institution experience over 13 years. Hematology. 2018;23(1):17-24.
doi pubmed - Ohanian M, Faderl S, Ravandi F, Pemmaraju N, Garcia-Manero G, Cortes J, Estrov Z. Is acute myeloid leukemia a liquid tumor? Int J Cancer. 2013;133(3):534-543.
doi pubmed - Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2(5):309-316.
doi pubmed - Ngu IW, Sinclair EC, Greenaway S, Greenberg ML. Unusual presentation of granulocytic sarcoma in the breast: a case report and review of the literature. Diagn Cytopathol. 2001;24(1):53-57.
doi - Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, Bennett JM. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48(6):1426-1437.
doi - Struhal W, Oberndorfer S, Lahrmann H, Lindeck-Pozza E, Hess B, Nussgruber V, Pohnl R, et al. Myeloid sarcoma in the central nervous system: case report and review of the literature. Acta Clin Croat. 2008;47(1):19-24.
- Dusenbery KE, Howells WB, Arthur DC, Alonzo T, Lee JW, Kobrinsky N, Barnard DR, et al. Extramedullary leukemia in children with newly diagnosed acute myeloid leukemia: a report from the Children's Cancer Group. J Pediatr Hematol Oncol. 2003;25(10):760-768.
doi pubmed - Samborska M, Derwich K, Skalska-Sadowska J, Kurzawa P, Wachowiak J. Myeloid sarcoma in children - diagnostic and therapeutic difficulties. Contemp Oncol (Pozn). 2016;20(6):444-448.
doi pubmed - Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236(4803):799-806.
doi pubmed - Li Z, Stolzel F, Onel K, Sukhanova M, Mirza MK, Yap KL, Borinets O, et al. Next-generation sequencing reveals clinically actionable molecular markers in myeloid sarcoma. Leukemia. 2015;29(10):2113-2116.
doi pubmed - Pastoret C, Houot R, Llamas-Gutierrez F, Boulland ML, Marchand T, Tas P, Ly-Sunnaram B, et al. Detection of clonal heterogeneity and targetable mutations in myeloid sarcoma by high-throughput sequencing. Leuk Lymphoma. 2017;58(4):1008-1012.
doi pubmed - Shallis RM, Gale RP, Lazarus HM, Roberts KB, Xu ML, Seropian SE, Gore SD, et al. Myeloid sarcoma, chloroma, or extramedullary acute myeloid leukemia tumor: A tale of misnomers, controversy and the unresolved. Blood Rev. 2021;47:100773.
doi pubmed - Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid sarcoma: presentation, diagnosis, and treatment. Clin Lymphoma Myeloma Leuk. 2017;17(5):263-267.
doi pubmed - Pui MH, Fletcher BD, Langston JW. Granulocytic sarcoma in childhood leukemia: imaging features. Radiology. 1994;190(3):698-702.
doi pubmed - Stolzel F, Rollig C, Radke J, Mohr B, Platzbecker U, Bornhauser M, Paulus T, et al. 18F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica. 2011;96(10):1552-1556.
doi pubmed - Cunningham I, Kohno B. 18 FDG-PET/CT: 21st century approach to leukemic tumors in 124 cases. Am J Hematol. 2016;91(4):379-384.
doi pubmed - Aschoff P, Hantschel M, Oksuz M, Werner MK, Lichy M, Vogel W, Pfannenberg C. Integrated FDG-PET/CT for detection, therapy monitoring and follow-up of granulocytic sarcoma. Initial results. Nuklearmedizin. 2009;48(5):185-191.
doi pubmed - Mora J, Cruz O, Tuset E, del Mar Perez M. Primitive hematopoietic malignant neoplasm presenting as a CD43-positive, small round, blue-cell tumor in an infant. Pediatr Blood Cancer. 2005;45(6):865-866.
doi pubmed - Traweek ST, Arber DA, Rappaport H, Brynes RK. Extramedullary myeloid cell tumors. An immunohistochemical and morphologic study of 28 cases. Am J Surg Pathol. 1993;17(10):1011-1019.
doi pubmed - Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785-3793.
doi pubmed - Mourad W, Kfoury H, Al Husseini H. The value of CD34, myeloperoxidase and chloroacetate esterase (Leder) stain in the diagnosis of granulocytic sarcoma. Ann Saudi Med. 2001;21(5-6):287-291.
doi pubmed - Ohanian M, Huang RS, Yakoushina TV, Estrov Z, Juneja H, Chen L, Idowu M, et al. Isolated mesenteric CD20-positive myeloid sarcoma. Clin Lymphoma Myeloma Leuk. 2014;14(6):e217-220.
doi pubmed - Alvarez P, Navascues CA, Ordieres C, Pipa M, Vega IF, Granero P, Alvarez JA, et al. Granulocytic sarcoma of the small bowel, greater omentum and peritoneum associated with a CBFbeta/MYH11 fusion and inv(16) (p13q22): a case report. Int Arch Med. 2011;4(1):3.
doi pubmed - Li YF, Zhang R, Zhang XH. [Granulocytic sarcoma of abdomen in an an acute myeloid leukemia patient with inv(16) and t(6;17) chromosome abnormalities: a case report and literature review]. Zhonghua Xue Ye Xue Za Zhi. 2011;32(5):342-343.
- Welch JS. Patterns of mutations in TP53 mutated AML. Best Pract Res Clin Haematol. 2018;31(4):379-383.
doi pubmed - Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2(1):a001008.
doi pubmed - Barbosa K, Li S, Adams PD, Deshpande AJ. The role of TP53 in acute myeloid leukemia: Challenges and opportunities. Genes Chromosomes Cancer. 2019;58(12):875-888.
doi pubmed - Cerquozzi S, Tefferi A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 2015;5:e366.
doi pubmed - Naesens L, Devos H, Nollet F, Michaux L, Selleslag D. Mediastinal myeloid sarcoma with TP53 mutation preceding acute myeloid leukemia with a PICALM-MLLT10 fusion gene. Acta Haematol. 2018;140(2):97-104.
doi pubmed - Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, Di Giandomenico S, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4(1):37-48.
doi pubmed - TeKippe M, Harrison DE, Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol. 2003;31(6):521-527.
doi - Chen J, Ellison FM, Keyvanfar K, Omokaro SO, Desierto MJ, Eckhaus MA, Young NS. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008;36(10):1236-1243.
doi pubmed - Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53-/-p16Ink4a-/-p19Arf-/- multipotent progenitors. Nature. 2008;453(7192):228-232.
doi pubmed - Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994;8(1):66-69.
doi pubmed - Chang H, Brandwein J, Yi QL, Chun K, Patterson B, Brien B. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28(10):1007-1011.
doi pubmed - Seymour JF, Pierce SA, Kantarjian HM, Keating MJ, Estey EH. Investigation of karyotypic, morphologic and clinical features in patients with acute myeloid leukemia blast cells expressing the neural cell adhesion molecule (CD56). Leukemia. 1994;8(5):823-826.
- Ganzel C, Manola J, Douer D, Jacob M, Rowe HF, Fernandez EM, et al. Extramedullary disease in adult acute myeloid leukemia is common but lacks independent significance: analysis of patients in ECOG-ACRIN cancer research group trials. J Clin Oncol. 2017;35(2):263.
- Junca J, Garcia-Caro M, Granada I, Rodriguez-Hernandez I, Torrent A, Morgades M, Ribera JM, et al. Correlation of CD11b and CD56 expression in adult acute myeloid leukemia with cytogenetic risk groups and prognosis. Ann Hematol. 2014;93(9):1483-1489.
doi pubmed - Graf M, Reif S, Kroll T, Hecht K, Nuessler V, Schmetzer H. Expression of MAC-1 (CD11b) in acute myeloid leukemia (AML) is associated with an unfavorable prognosis. Am J Hematol. 2006;81(4):227-235.
doi pubmed - Zebisch A, Wolfler A, Fried I, Wolf O, Lind K, Bodner C, Haller M, et al. Frequent loss of RAF kinase inhibitor protein expression in acute myeloid leukemia. Leukemia. 2012;26(8):1842-1849.
doi pubmed - Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, Thomas DA, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17(6):1100-1103.
doi pubmed - Shahin OA, Ravandi F. Myeloid sarcoma. Curr Opin Hematol. 2020;27(2):88-94.
doi pubmed - Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340-350.
doi pubmed - Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002;94(6):1739-1746.
doi pubmed - Antic D, Elezovic I, Milic N, Suvajdzic N, Vidovic A, Perunicic M, Djunic I, et al. Is there a "gold" standard treatment for patients with isolated myeloid sarcoma? Biomed Pharmacother. 2013;67(1):72-77.
doi pubmed - Lazzarotto D, Candoni A, Fili C, Forghieri F, Pagano L, Busca A, Spinosa G, et al. Clinical outcome of myeloid sarcoma in adult patients and effect of allogeneic stem cell transplantation. Results from a multicenter survey. Leuk Res. 2017;53:74-81.
doi pubmed - Chevallier P, Mohty M, Lioure B, Michel G, Contentin N, Deconinck E, Bordigoni P, et al. Allogeneic hematopoietic stem-cell transplantation for myeloid sarcoma: a retrospective study from the SFGM-TC. J Clin Oncol. 2008;26(30):4940-4943.
doi pubmed - Chevallier P, Labopin M, Cornelissen J, Socie G, Rocha V, Mohty M, EBMT Ao. Allogeneic hematopoietic stem cell transplantation for isolated and leukemic myeloid sarcoma in adults: a report from the Acute Leukemia Working Party of the European group for Blood and Marrow Transplantation. Haematologica. 2011;96(9):1391-1394.
doi pubmed - Shimizu H, Saitoh T, Tanaka M, Mori T, Sakura T, Kawai N, Kanda Y, et al. Allogeneic hematopoietic stem cell transplantation for adult AML patients with granulocytic sarcoma. Leukemia. 2012;26(12):2469-2473.
doi pubmed - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - Anderson MA, Deng J, Seymour JF, Tam C, Kim SY, Fein J, Yu L, et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood. 2016;127(25):3215-3224.
doi pubmed - Granowicz EM, Jonas BA. Targeting TP53-mutated acute myeloid leukemia: research and clinical developments. Onco Targets Ther. 2022;15:423-436.
doi pubmed - Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, Cluzeau T, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol. 2021;39(14):1584-1594.
doi pubmed - Unglaub JM, Schlenk RF, Hanooun M, Reinhardt HC. Venetoclax-azacitidine as salvage therapy and bridge to allogeneic cell transplantation in relapsed/refractory AML compared to historical data of the SAL registry study. Blood. 2021;138(Supplement 1):4418.
doi - Piccaluga PP, Martinelli G, Rondoni M, Malagola M, Gaitani S, Isidori A, Bonini A, et al. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymphoma. 2004;45(9):1791-1795.
doi pubmed - Vedy D, Muehlematter D, Rausch T, Stalder M, Jotterand M, Spertini O. Acute myeloid leukemia with myeloid sarcoma and eosinophilia: prolonged remission and molecular response to imatinib. J Clin Oncol. 2010;28(3):e33-35.
doi pubmed - Sallman DA, Elmariah H, Sweet KL, Talati C, Mishra A, Kelley LL, Lankford A, et al. A phase 1/1b safety study of Prgn-3006 Ultracar-T™ in patients with relapsed or refractory CD33-positive acute myeloid leukemia and higher risk myelodysplastic syndrome. Blood. 2020;136(Supplement 1):17.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


