| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 9, September 2022, pages 475-481
Polyethylene Glycol 3350 Crystal Nephropathy in Association With Glomerular Mesangial Immunoglobin A Deposition
Rasha Alya, Ratna Acharyab, Xu Zengc, Kiran Upadhyaya, d
aDivision of Pediatric Nephrology, Department of Pediatrics, University of Florida, Gainesville, FL, USA
bDivision of General Pediatrics, Department of Pediatrics, University of Florida, Gainesville, FL, USA
cDivision of Anatomic Pathology, Department of Pathology, University of Florida, Gainesville, FL, USA
dCorresponding Author: Kiran Upadhyay, Division of Pediatric Nephrology, Department of Pediatrics, University of Florida, Gainesville, FL 32610, USA
Manuscript submitted August 23, 2022, accepted September 22, 2022, published online September 28, 2022
Short title: PEG 3350 Oxalate Nephropathy and Mesangial IgA
doi: https://doi.org/10.14740/jmc4000
| Abstract | ▴Top |
Polyethylene glycol (PEG) 3350, an active ingredient of over-the-counter MiraLAX, is a commonly used laxative in children and is produced by polymerization of ethylene glycol (EG). Masked EG toxicity secondary to contamination of PEG 3350 could occur. We present a 7-year-old child with developmental delay who presented with altered mental status and acute kidney injury (AKI) following intake of generic PEG 3350 for few days prior to presentation. There was high anion gap metabolic acidosis, hypernatremia, elevated osmolar gap, lactic acidosis, and AKI. Urinalysis showed tubular proteinuria, microscopic hematuria, and calcium oxalate crystals. Prior urinalyses were normal without hematuria or proteinuria. Renal biopsy revealed evidence of mesangial dominant immunoglobulin A (IgA) and complement 3 (C3) deposits along with dense tubular deposition of calcium oxalate crystals. He subsequently developed worsening oliguric AKI and required hemodialysis (HD) for several sessions. The AKI resolved within 2 weeks and further HD was not required. Mental status improved in few days. Follow-up urinalyses showed resolution of microscopic hematuria and crystalluria. We hypothesized that the generic PEG 3350 most likely was contaminated with EG leading to the presentation. A high index of suspicion of contamination of PEG 3350 with EG is required in patients presenting with unexplained high anion gap metabolic acidosis, elevated osmolar gap, lactic acidosis, AKI, calcium oxalate crystalluria, and oxalate crystals on renal biopsy. Further studies are needed to determine whether there is an association between transient glomerular mesangial IgA deposition and crystal nephropathy.
Keywords: Polyethylene glycol 3350; Acute kidney injury; Mesangial; IgA; Oxalate
| Introduction | ▴Top |
Polyethylene glycol (PEG) is a hydrophilic polyether compound derived from petroleum and has been used in numerous applications from industrial manufacturing to biomedicines [1]. It has been used in multiple pharmaceutical products in topical, oral, and parenteral forms such as cosmetics, skin creams, toothpastes, laxatives, and recently as an excipient in both the Moderna and Pfizer-BioNTech vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. PEG with an average molecular weight of 3,350 daltons, known as PEG 3350, is the active ingredient of MiraLAX. MiraLAX, an osmotic laxative, was first approved as a prescription laxative in February 1999, and then approved as over-the-counter medication in May 2019 [3]. PEG is produced by the interaction of ethylene oxide with water, ethylene glycol (EG), or ethylene glycol oligomers. EG and its oligomers are more preferred than water, because of its low molecular weight of 62 g/mol that allow production of narrow molecular weight distribution [4]. EG, a common component of anti-freeze and deicer solutions, is known to cause oxalate nephropathy. EG toxicity is secondary to the metabolic acidosis resulting from the biotransformation of EG into toxic metabolites (Fig. 1). Glycolic acid causes severe acidosis and oxalate precipitates as calcium oxalate monohydrate (COM) in the renal tubules, resulting in oxalate nephropathy [5].
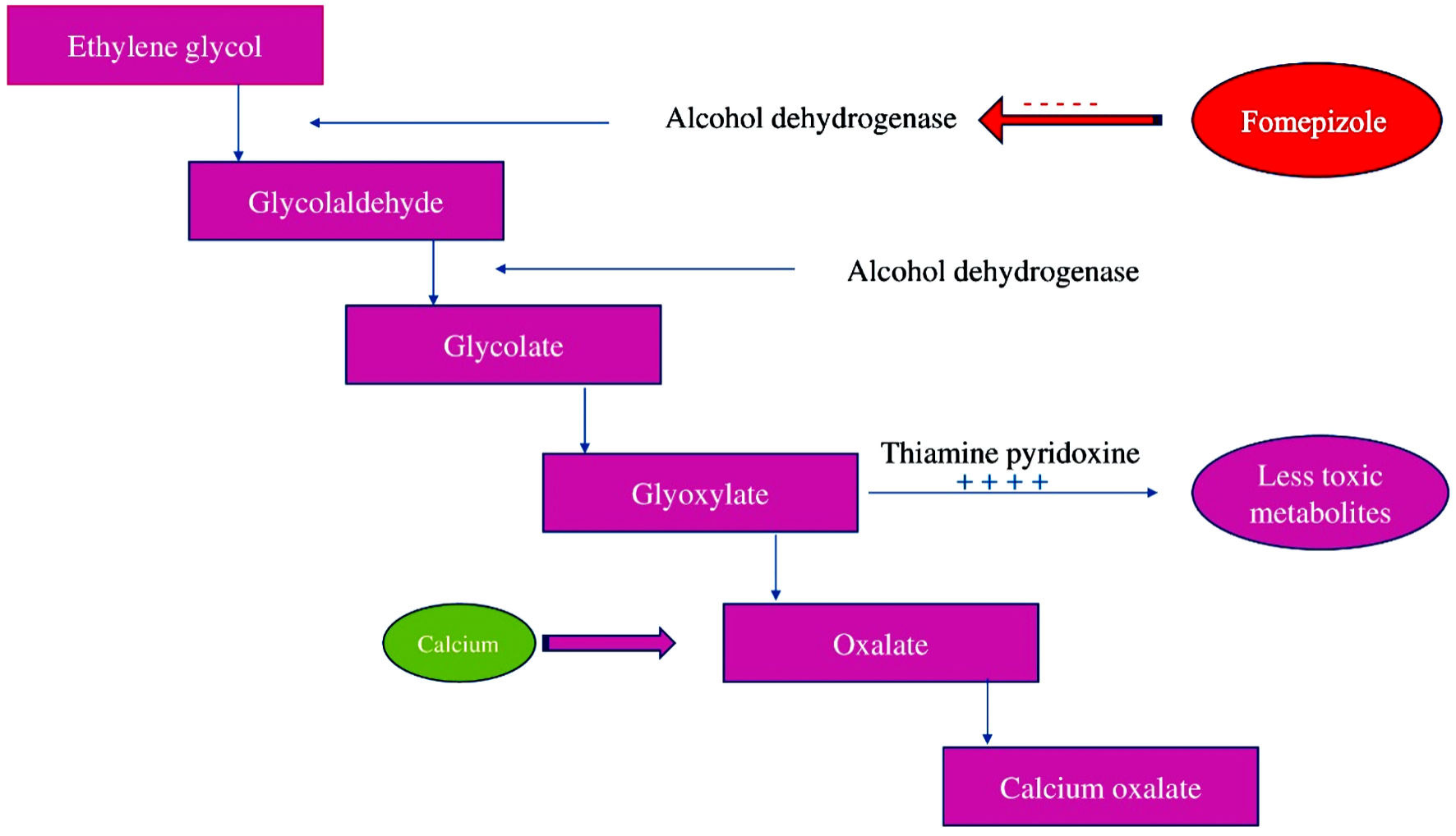 Click for large image | Figure 1. Ethylene glycol metabolism. |
We present a case of acute oxalate nephropathy most likely secondary to consumption of contaminated PEG 3350 with EG, in a child without a known prior kidney disease along with incidental finding of mesangial dominant immunoglobulin A (IgA) and complement 3 (C3) deposits. To the best of our knowledge, the association of oxalate nephropathy with mesangial IgA deposits has not been reported.
| Case Report | ▴Top |
Investigations
A 7-year-old male was seen in the emergency department for progressive lethargy for 1 day. There was no history of fever, vomiting, diarrhea, dysuria, gross hematuria, dark brown urine, recent decrease in the urine output or appearance of new rash. There was no history of recent infections, inflammatory bowel disease, celiac disease, lung disease or liver disease in the past. There was no history of autoimmune disorders such as ankylosing spondylitis, Sjogrens syndrome, rheumatoid arthritis, psoriasis, or malignancies. He had a history of chronic constipation that was managed with MiraLAX. His past medical history was remarkable for prematurity at 28 weeks gestation, gastroparesis, cerebral palsy, hydrocephalus, and complex partial seizure which was well controlled on oxcarbazepine and levetiracetam. His surgical history included ventriculoperitoneal (VP) shunt and fourth ventricular stent placement at the age of 2 months of life, and Nissen fundoplication with gastrostomy tube (G-tube) placement at the age of 1 year. Family history was not suggestive of autoimmune diseases, renal failure, dialysis, or kidney transplantation. His dietary regimen consisted of continuous Kate Farms formula via G-tube. There was no history of excessive dietary oxalate consumption. His medications included oxcarbazepine, levetiracetam, pantoprazole and MiraLAX. He was started on a new bottle of generic PEG 3350 a few days ago. He had a history of baseline developmental delay and was non-verbal but was able to laugh, smile, wave and usually was in bright mood prior to the presentation.
On admission, he appeared ill, and his vital signs showed temperature 37.1 °C (98.8 °F), heart rate 129 beats per minute, respiratory rate 27 per minute, blood pressure 128/66 mm Hg, oxygen saturation of 100 % on room air, weight 34.9 kg (77 lb), height 1.34 m and body mass index 19.44 kg/m2. Physical examination showed a lethargic child with Kussmaul breathing and altered mental status. Physical examination showed a non-verbal and wheelchair-bound child. He appeared to be in some distress. He had normal conjunctiva, pupils were equal, round, and reactive to light with a blank stare. There was well-healed prior tracheostomy scar. Chest examination showed tachypnea without nasal flaring, wheezing or rales. Cardiovascular examination showed tachycardia with regular rhythm, normal heart sounds, no murmurs or gallop. Abdomen was soft with normal bowel sounds, and no mass, tenderness, or distension. There was a G-tube in place with dry and clean dressing. Skin was warm and dry without cyanosis, erythema, or rash. Capillary refill time was less than 2 s. He had bilateral upper and lower extremities contractures. He had altered mental status with minimal movements and unable to follow commands. He was admitted in the intensive care unit due to high anion gap metabolic acidosis, elevated osmolar gap, hypernatremia, and acute kidney injury (AKI).
An initial venous blood gas analysis showed: pH 7.05, PaCO2 24 mm Hg, PaO2 33 mm Hg, and bicarbonate 7 mmol/L. Serum lactate was elevated at 5.8 mmol/L. Initial serum chemistry showed sodium 154 mmol/L, potassium 4.7 mmol/L, chloride 120 mmol/L, bicarbonate 7 mmol/L, calcium 9.9 mg/dL, albumin 3.9 g/dL, phosphorus 5.8 mg/dL, glucose 132 mg/dL, blood urea nitrogen 22 mg/dL, creatinine 1.3 mg/dL (Table 1). His baseline serum creatinine a week prior was 0.5 mg/dL which increased to a peak value of 6.1 mg/dL after 2 days of admission. Measured serum osmolality was 352 mOsm/kg H2O. Calculated serum osmolality was 323 mOsm/kg H2O. Serum osmolal gap was 29 mOsm/kg H2O. White blood cell count showed total white count of 9.1 × 109/L, hemoglobin 13.1 g/dL, and platelet count of 470 × 109/L. Urine electrolytes showed urine sodium of 51 mmol/L and osmolality of 145 mOsm/kg H2O. Urinalysis showed specific gravity 1.009, pH 5, 23 red cells per high power field, absence of protein and ketones, and plenty of calcium oxalate crystals. Random urine protein creatinine ratio was 0.9 mg/mg, mainly tubular proteinuria with elevated urine β2-microgloblin 10,252 µg/L (normal < 300 µg/L). Random urine calcium creatinine ratio was normal at 0.04 mg/mg. Liver function test showed stable transaminases. Hepatitis profile was negative.
 Click to view | Table 1. Laboratory Values From the Initial Presentation to Until 24 H After Presentation |
Renal bladder sonogram showed 9.8 cm and 9.6 cm kidneys with normal echogenicity and without hydronephrosis. Other investigations showed normal serum immunoglobulins (Ig), including IgA. Brain natriuretic peptide was normal at 41 pg/mL. Serum complements C3 and C4 were normal. Antinuclear antibody was 1:160 in the homogenous pattern. Anti-double stranded DNA was negative. Anti-neutrophil cytoplasmic antibodies and anti-glomerular basement membrane antibodies were negative. Anti-streptolysin O was 216 IU/mL (normal: < 200 IU/mL). C-reactive protein was 36.5 mg/dL. Procalcitonin was 0.53 ng/mL. Blood culture was negative. Respiratory viral polymerase chain reaction (PCR) including SARS-CoV-2 was negative. Epstein-Barr virus (EBV) and cytomegalovirus (CMV) DNA PCRs were negative. Serum creatine kinase was 50 U/L (normal 0 - 200 U/L). Review of prior urinalyses showed absence of microscopic hematuria and proteinuria. X-ray abdomen showed moderate amount of stool in the colon. VP shunt series was stable. A non-contrast computed tomography of the head showed no acute intracranial hemorrhage, regional mass effect, midline shift or herniation. Electroencephalogram showed diffuse slowing but no epileptiform discharge.
Urine toxicology was negative. Serum salicylate level and ethyl alcohol levels were below detectable limits. Serum EG and propylene glycol levels were not measured. We were also not able to obtain the bottle of generic PEG 3350 and send it for testing to confirm contamination with EG.
Diagnosis
A percutaneous renal biopsy obtained a week after admission showed three portions of cortical tissue with 21 glomeruli on light microscopy with mild to moderate increase in glomerular mesangial cellularity without endocapillary proliferation, segmental sclerosis, necrosis, or crescents (Fig. 2). Most of the proximal and distal tubular cells showed yellowish white, radially arranged crystals with birefringence under polarization, consistent with calcium oxalate crystals with a layer of CD68 positive mononuclear cells outside the crystals, representing macrophages (Fig. 3). There was mild interstitial CD3 positive lymphocytic inflammation. There was no acute tubular necrosis. Immunofluorescence showed diffuse granular mesangial staining for predominant IgA (3+) and C3 (1+) (Fig. 2). There was no specific glomerular staining for IgG, IgM, C1Q or kappa light chain. Electron microscopy showed normal trilaminar structure with mesangial electron-dense immune deposits without subendothelial or subepithelial immune deposits, along with intact podocyte foot processes.
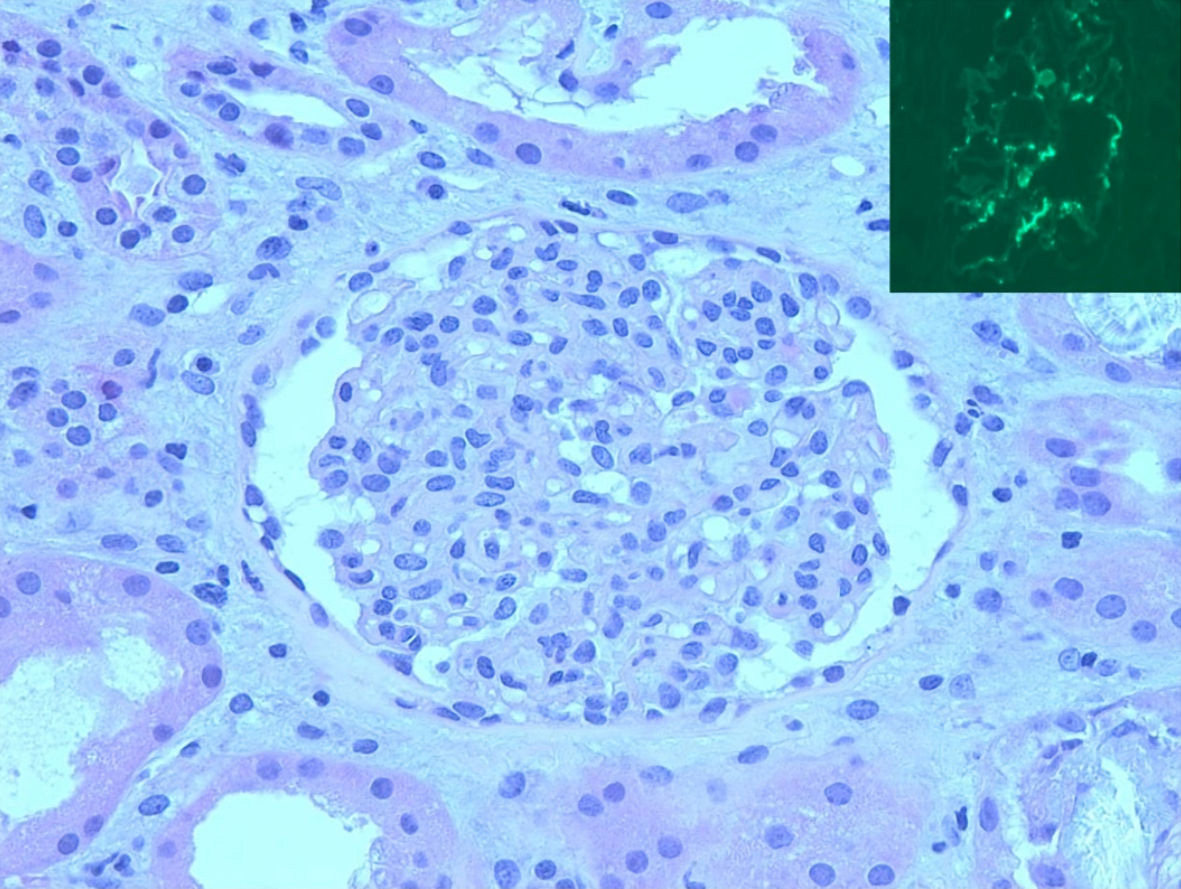 Click for large image | Figure 2. Kidney biopsy. Light microscopy (H&E, 40 × 10) showed mesangial hypercellular glomerulus. Inset: mesangial IgA staining by immunofluorescence. H&E: hematoxylin and eosin; immunoglobulin A. |
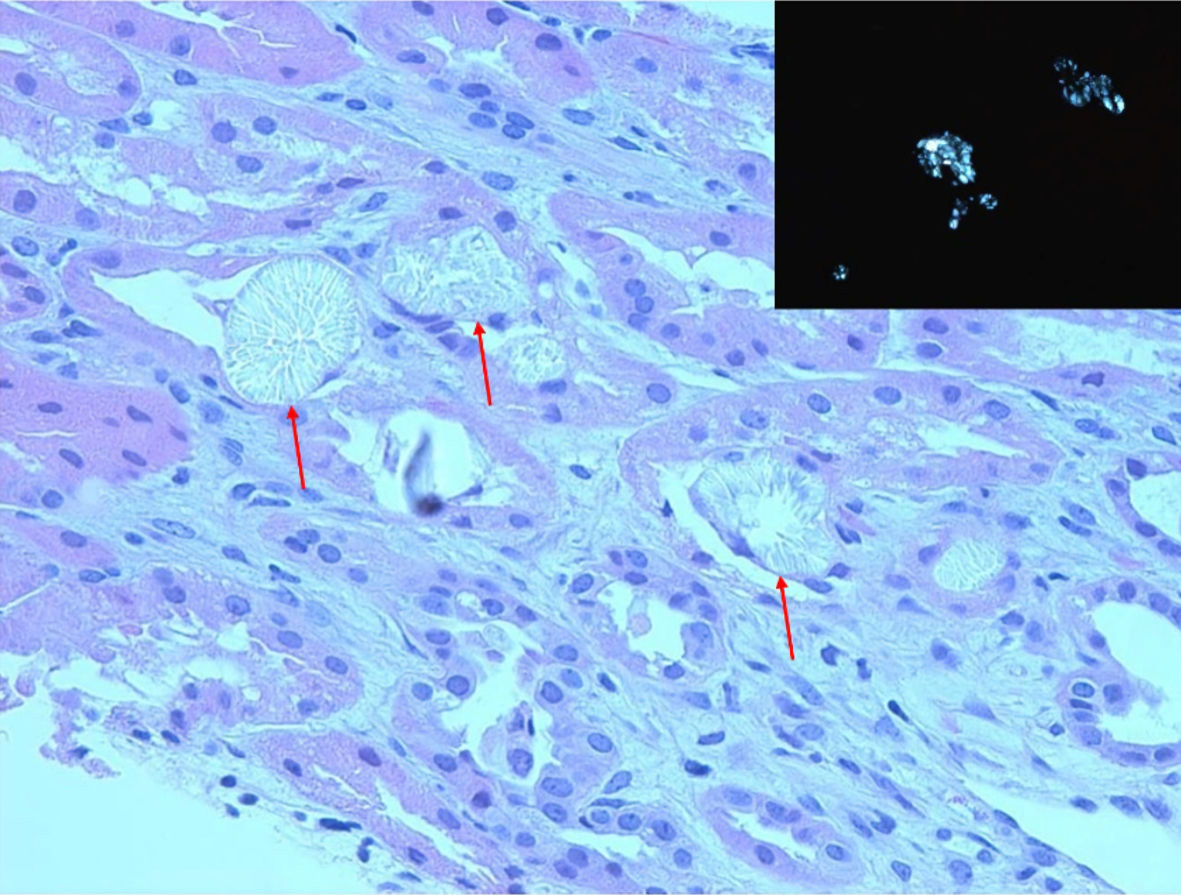 Click for large image | Figure 3. Kidney biopsy. Light microscopy (H&E, 20 × 10) showed proximal and distal tubules containing extensive yellowish-white, radially arranged crystals with cellular reaction (red arrows). Inset: birefringent crystals under polarization, consistent with calcium oxalate crystals. H&E: hematoxylin and eosin. |
Treatment
Patient received multiple intravenous (IV) fluid boluses and was started on bicarbonate containing IV fluid. Given urine microscopy finding of oxalate crystals, there was a concern for an EG ingestion. Mother had reported that only new medication was a generic PEG 3350 that she had obtained from a local store and his brother had vomiting episodes after receiving the same PEG 3350, raising concern for possible contamination of PEG 3350 by EG. There was no history of exposure to antifreeze, and accidental ingestion of other household agents. Due to concern for EG, propylene glycol, or other alcohol vs. other toxic ingestion, poison control was consulted. He received thiamine, folic acid, and pyridoxine for neuroprotection.
The patient subsequently developed oliguria. Due to persistent metabolic acidosis, oligoanuria and concern for toxic ingestion, emergent hemodialysis (HD) was started on day 2 of admission. Acidosis and sensorium improved with HD. Ethanol and/or fomepizole therapy was not administered. Fomepizole was thought to be unnecessary by the treatment team due to the severe renal impairment and the need for HD, along with the absence of serum EG level. His diet was changed to Suplena from Kate Farms. He did not have seizures. He intermittently had hypertension suggesting fluid overload that improved with dialysis.
Follow-up and outcomes
Tenth day after admission, he started to void spontaneously, and his renal function started improving with stable electrolytes and serum creatinine of 2.8 mg/dL. HD was discontinued. His mental status returned to baseline. After discharge, weekly follow-up renal function test showed gradual improvement of the serum creatinine to 0.7 mg/dL within 3 weeks of discharge. Renal diet with Suplena was switched back to the original Kate Farms formula. Urinalysis did not show microscopic hematuria or crystalluria at 3-week follow-up. A repeat renal biopsy was not performed.
| Discussion | ▴Top |
The initial diagnosis of EG intoxication may be difficult in the absence of documented history of EG ingestion or in the absence of serum EG level, as in our case. However, even in the absence of these, a high index of suspicion for EG toxication is required in someone presenting with the initial findings of elevated osmolar gap, high anion gap metabolic acidosis, lactic acidosis, and AKI. Oxalate nephropathy is a well-known cause of AKI [6]. In a series by Karlson-Stiber et al, 50% of patients required renal replacement therapy, most had a renal recovery. Hence, early recognition and rapid treatment are needed to decrease the morbidity and mortality [7]. Calcium oxalate crystalluria and oxalate crystals in the renal biopsy further solidifies the diagnosis of EG intoxication.
Contamination of PEG 3350, a common osmotic laxative, with EG can occur. In 2008, the Food and Drug Administration reported contamination of eight batches of MiraLAX with small amounts of EG and diethylene glycol [8]. Our patient had been using a new bottle of PEG 3350 for few days prior to presentation and it is possible that it was contaminated with EG. Although our patient had all clinical and laboratory findings seen in EG intoxication, in the absence of serum EG level and documented history of EG intoxication, the diagnosis of EG contamination of PEG 3350 remains a mere speculation.
Lactic acidosis can occur in EG toxicity as an artifact due to the structural similarity of the lactate and glycolate molecules or a real finding due to inhibition of cellular metabolic enzymes by glycolate, leading to lactic acidosis [9, 10]. The mechanism of AKI is most likely secondary to crystallopathy. Acute supersaturation of calcium oxalate crystals leads to direct and indirect renal epithelial cytotoxicity, and inflammation-driven cell necrosis with resulting AKI. Later, persistent crystal deposition generates sub-acute crystal plug formation in distal tubules or collecting ducts leading to tubule obstruction and oligoanuria [11]. Studies in cultured human proximal tubule cells have demonstrated that only COM crystals, not the oxalate ion, glycolaldehyde, or glyoxylate, produce a necrotic cell death at toxicologically relevant concentrations [1]. COM crystals have been shown to alter the membrane structure and function, increase the reactive oxygen species and produce mitochondrial dysfunction [12]. In our case, despite severe AKI, tubular necrosis was not observed but could be due to a sampling bias.
The development of IgA nephropathy (IgAN) requires an autoantibody (IgG or IgA) against the autoantigen galactose-deficient (Gd) IgA1 which subsequently leads to the development of inflammatory and fibrotic renal damage due to deposition of anti-Gd IgA1 and Gd-IgA1 immune complexes in the glomerular mesangium [13]. The precise origin of poorly O-galactosylated IgA1 and the inciting factors to produce O-glycan-specific antibodies continues to be the subject of ongoing investigation. Total serum IgA can be elevated in up to 50% of cases of IgAN but is neither specific nor a sensitive biomarker [14]. Primary IgAN is manifested by dominant or co-dominant IgA deposits in the glomeruli, usually accompanied by C3, IgG and IgM [15]. Secondary IgAN can be associated with liver disease, infections, mucosal inflammation, celiac disease, inflammatory bowel disease, other autoimmune conditions, and neoplasia [16, 17]. The case described in this report had none of these conditions except for chronic constipation. It is possible that gastrointestinal mucosal alterations and dysbiosis of gut microbiota secondary to chronic constipation can activate the innate immune system, aggravating the pre-existent IgA nephropathy and promoting disease manifestations such as microscopic hematuria [18]. Indeed, it has been shown that the dysregulation of the interplay between intestinal immunity, diet and gut microbiota can lead to the production of O-galactosylated IgA [19]. However, it is important to mention that mesangial IgA deposition has been described in asymptomatic individuals and the clinical significance of such finding is not very clear [20]. In kidney transplant recipients, latent mesangial IgA deposition does not correlate with disease progression [21]. This patient could have had preexisting mesangial IgA deposits and might have been an incidental detection in the kidney biopsy. However, the new onset of microscopic hematuria during this clinical course and subsequent resolution during recovery is suggestive of transient exacerbation of the IgAN. In fact, transient IgAN with dialysis-requiring AKI has been described in dengue fever with complete reversal of glomerular changes a few months later [22]. Also, it has been reported that the repeat biopsy of patients with IgAN on clinical remission showed improvement of the glomerular changes on light microscopy, disappearance, or diminution of IgA deposits in the mesangium and decreased electron-dense deposits [23]. In our patient, due to normalization of renal function and urine findings, a repeat renal biopsy was not thought to be necessary.
Anti-PEG antibodies have been shown to contribute to the complement activation [24]. Complements have been shown to have a role in the pathogenesis of IgAN, mainly the alternative and lectin complement pathways [25]. Low levels of anti-PEG antibodies are commonly seen due to chronic environmental exposure or in those with history of chronic usage of PEG. Our patient had been on chronic PEG 3350 therapy, and we hypothesized that the anti-PEG antibodies contributed to the development of C3 deposition and probable complement-induced nephropathy. This needs to be studied in future studies. We did not measure the anti-PEG antibodies and is a limitation of this report. Increased levels of IgM and IgG PEG antibodies have also been shown in people who have received SARS-CoV-2 mRNA vaccine which contains PEG as an excipient [26]. Our patient had completed two doses of vaccine several months prior to the presentation.
Whether there is an association between tubular oxalate deposition and mesangial IgA deposits is not clear. Ting et al described two adults with EG poisoning who had classic features of oxalate nephropathy but without any glomerular IgA or complement deposition [10]. Similarly, other studies of oxalate nephropathy have not demonstrated co-existent mesangial IgA deposits. This again is an area of further studies.
Limitations of this report include absence of serum EG level, no proof of definite contamination of PEG 3350 by EG, absence of usage of fomepizole when there was more than one evidence of suspected EG intoxication and absence of baseline renal biopsy prior to presentation and follow-up renal biopsy.
Learning points
EG toxicity may occur without a clear history of ingestion. PEG 3350, a common laxative, may be contaminated with EG. Hence, a detailed history and appropriate investigations are useful to establish a definite diagnosis. EG poisoning is life-threatening and requires rapid recognition by careful evaluation of findings such as elevated osmolar gap, high anion gap metabolic acidosis, lactic acidosis, hypocalcemia, and urinary calcium oxalate crystals along with calcium oxalate crystal deposition in the renal tubules. Hemodialysis may be indicated for severe AKI. Mesangial IgA deposition in our patient was an interesting association and will need to be studied further.
Conclusions
This report illustrates a case of transient acute oxalate nephropathy in the setting of intake of possibly EG-contaminated PEG 3350, a very commonly prescribed laxative, in a patient without known pre-existent kidney disease. Incidentally, there was a mesangial IgA deposition as well. Given the widespread usage of this laxative in the general population, this case warrants suspicion of EG contamination in patients with abrupt onset AKI of unclear etiology.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
We obtained written informed consent from the parent of the patient for publication of this case report.
Author Contributions
Rasha Aly was in charge of writing the case and structure/flow of the paper. Ratna Acharya was in charge of writing the conception and discussion. Xu Zeng was in charge of writing the pathology discussion and interpretation of the biopsy. Kiran Upadhyay oversaw the concept, writing, and edition of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AKI: acute kidney injury; C3: complement 3; C4: complement 4; CMV: cytomegalovirus; COM: calcium oxalate monohydrate; EBV: Epstein-Barr virus; EG: ethylene glycol; G-tube: gastrostomy tube; HD: hemodialysis; IgA: immunoglobulin A; IgAN: IgA nephropathy; IV: intravenous; PCR: polymerase chain reaction; PEG: polyethylene glycol; VP: ventriculoperitoneal
| References | ▴Top |
- Kahovec J, Fox RB, Hatada K. Nomenclature of regular single-strand organic polymers- IUPAC recommendations. Pure and Applied Chemistry. 2002;74(10):1921-1956.
doi - Cabanillas B, Akdis CA, Novak N. Allergic reactions to the first COVID-19 vaccine: A potential role of polyethylene glycol? Allergy. 2021;76(6):1617-1618.
doi pubmed - American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211-217.
doi pubmed - Tonini M. Polyethylene glycol as a non-absorbable prokinetic agent in the lower gastrointestinal tract. Ital J Gastroenterol Hepatol. 1999;31(Suppl 3):S238-241.
- McMartin K. Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning? Clin Toxicol (Phila). 2009;47(9):859-869.
doi pubmed - Buysschaert B, Aydin S, Morelle J, Gillion V, Jadoul M, Demoulin N. Etiologies, clinical features, and outcome of oxalate nephropathy. Kidney Int Rep. 2020;5(9):1503-1509.
doi pubmed - Karlson-Stiber C, Persson H. Ethylene glycol poisoning: experiences from an epidemic in Sweden. J Toxicol Clin Toxicol. 1992;30(4):565-574.
doi pubmed - https://www.nytimes.com/2015/01/06/science/scrutiny-for-a-childhood-remedy.html.
- Verelst S, Vermeersch P, Desmet K. Ethylene glycol poisoning presenting with a falsely elevated lactate level. Clin Toxicol (Phila). 2009;47(3):236-238.
doi pubmed - Ting SM, Ching I, Nair H, Langman G, Suresh V, Temple RM. Early and late presentations of ethylene glycol poisoning. Am J Kidney Dis. 2009;53(6):1091-1097.
doi pubmed - Mulay SR, Anders HJ. Crystal nephropathies: mechanisms of crystal-induced kidney injury. Nat Rev Nephrol. 2017;13(4):226-240.
doi pubmed - McMartin KE, Wallace KB. Calcium oxalate monohydrate, a metabolite of ethylene glycol, is toxic for rat renal mitochondrial function. Toxicol Sci. 2005;84(1):195-200.
doi pubmed - Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71(11):1148-1154.
doi pubmed - van der Boog PJ, van Kooten C, van Seggelen A, Mallat M, Klar-Mohamad N, de Fijter JW, Daha MR. An increased polymeric IgA level is not a prognostic marker for progressive IgA nephropathy. Nephrol Dial Transplant. 2004;19(10):2487-2493.
doi pubmed - Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402-2414.
doi pubmed - Saha MK, Julian BA, Novak J, Rizk DV. Secondary IgA nephropathy. Kidney Int. 2018;94(4):674-681.
doi pubmed - Long JA, Cook WJ. IgA deposits and acute glomerulonephritis in a patient with staphylococcal infection. Am J Kidney Dis. 2006;48(5):851-855.
doi pubmed - Zhao Y, Yu YB. Intestinal microbiota and chronic constipation. Springerplus. 2016;5(1):1130.
doi pubmed - Monteiro RC. Recent advances in the physiopathology of IgA nephropathy. Nephrol Ther. 2018;14(Suppl 1):S1-S8.
doi pubmed - Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63(6):2286-2294.
doi pubmed - Hara S, Ichimaru N, Kyo M, Yamaguchi Y, Kojima Y, Takahara S, Itoh T. Latent mesangial immunoglobulin A deposition in long-term functioning kidney does not correlate with disease progression and may exhibit fluctuating patterns. Transplant Proc. 2014;46(1):124-129.
doi pubmed - Upadhaya BK, Sharma A, Khaira A, Dinda AK, Agarwal SK, Tiwari SC. Transient IgA nephropathy with acute kidney injury in a patient with dengue fever. Saudi J Kidney Dis Transpl. 2010;21(3):521-525.
- Yoshikawa N, Iijima K, Matsuyama S, Suzuki J, Kameda A, Okada S, Nakamura H. Repeat renal biopsy in children with IgA nephropathy. Clin Nephrol. 1990;33(4):160-167.
- Neun BW, Barenholz Y, Szebeni J, Dobrovolskaia MA. Understanding the role of Anti-PEG antibodies in the complement activation by Doxil in vitro. Molecules. 2018;23(7):1700.
doi pubmed - Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J. Current Understanding of the Role of Complement in IgA Nephropathy. J Am Soc Nephrol. 2015;26(7):1503-1512.
doi pubmed - Ju Y, Lee WS, Pilkington EH, Kelly HG, Li S, Selva KJ, Wragg KM, et al. Anti-PEG Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine. ACS Nano. 2022.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


