| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 10, October 2022, pages 513-516
Statin-Induced Necrotizing Autoimmune Myositis
Yonatan Akivisa , Meenakshi Kurupb, Sabu Johna, c, d
aDepartment of Internal Medicine, Division of Cardiovascular Medicine, SUNY Downstate-Health Science University, Brooklyn, NY 11203, USA
bNewcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK
cKings County Hospital Center, Brooklyn, NY 11203, USA
dCorresponding Author: Sabu John, College of Medicine, Department of Medicine, Division of Cardiovascular Medicine, Downstate-Health Science University, Brooklyn, NY 11203-2098, USA
Manuscript submitted September 20, 2022, accepted October 15, 2022, published online October 31, 2022
Short title: SINAM
doi: https://doi.org/10.14740/jmc4010
| Abstract | ▴Top |
Statins are the most frequently prescribed medications for primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD). The United States Preventative Services Task Force recommends that clinicians selectively offer a statin for the primary prevention of ASCVD for adults aged 40 - 75 years with one or more cardiovascular disease risk factors and an estimated 10-year risk of a cardiovascular event of 10% or greater. Despite their ubiquity, it is estimated that approximately 6-10% of patients remain intolerant due to muscle aches. Here, we present a case of a 71-year-old female that was taking atorvastatin for a year and presented to the emergency room with proximal muscle aches and weakness. Laboratory values were significant for an elevated creatinine kinase of 4,166 U/L (reference range, 20 - 180). Her magnetic resonance imaging was significant for edema in bilateral lower extremity proximal muscles. Serology revealed a high anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibody, confirming the diagnosis of statin-induced necrotizing autoimmune myositis. A muscle biopsy of the right vastus lateralis revealed necrotic muscle fibers. During her hospital course, she was treated with intravenous methylprednisolone, mycophenolate mofetil, and tacrolimus. Her symptoms gradually improved, and she was discharged after 14 days with a rheumatology follow-up. This is an exceedingly rare complication of statin use and has only recently received increasing attention. Here we present our experience with this disease.
Keywords: Statin-induced necrotizing autoimmune myositis; Statin; Myopathy; Anti-HMG-CoA
| Introduction | ▴Top |
Statins are one of the most widely prescribed medications because of their potent cholesterol level-lowering properties. In a meta-analysis published in the European Heart Journal in 2022, it is estimated that 6-10% of patients are intolerant to statins due to muscle aches [1]. Statin-associated myalgias are usually benign and resolve with cessation of the drug. However, a rare complication of statin use is statin-induced necrotizing autoimmune myositis (SINAM) [2]. SINAM is characterized by marked proximal muscle weakness, raised creatinine kinase (CK) levels (× 10 - 100 level), and the presence of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) autoantibodies. The pathogenesis of this disease is poorly understood. Still, it has been suggested that a subset of the population, such as patients with class II major histocompatibility complex (MHC) allele DRB1*11:01, are susceptible to developing anti-HMGCR autoantibodies after statin exposure [2-4]. Treatment includes immunosuppressive agents such as glucocorticoids, mycophenolate mofetil, methotrexate, and azathioprine [2-4]. In this case report, we describe our experience with SINAM.
| Case Report | ▴Top |
Investigations
The patient is a 71-year-old female with a past medical history of hypertension, diabetes mellitus type 2, and hyperlipidemia. She presented to the emergency room after 3 months of progressively worsening aches and weakness in her shoulders and thighs. The patient reported that she could not stand from a seated position nor lift her hands above her head. She endorsed that she had suffered multiple falls in the past month and could no longer ambulate without a cane. On review of systems, she complained of fatigue and recent hair loss. She denied fevers, headaches, vision changes, shortness of breath, chest pain, rashes, and dysuria. Home medications include lisinopril 5 mg, hydrochlorothiazide 25 mg, amlodipine 10 mg, metformin 1,000 mg, and atorvastatin 40 mg. She was started on atorvastatin a year before her presentation without requiring dose adjustments.
In the emergency department, she was afebrile with a temperature of 37.1 °C; her blood pressure was 131/55 mm Hg, pulse was 97 beats per minute, respiratory rate was 18 breaths per minute, and her oxygen saturation was 100% on room air. Electrocardiography (EKG) showed normal sinus rhythm with nonspecific ST and T wave changes. On physical examination, she was alert and oriented. Her lung sounds were clear bilaterally, with good air movement. Cardiac auscultation revealed tachycardia with regular rhythm and a crescendo decrescendo murmur at the right upper sternal border. Musculoskeletal examination revealed mild non-tender bogginess of metacarpophalangeal and proximal interphalangeal joints bilaterally. The dermatological examination was significant for a heliotrope rash. On neurological examination, she could not lift her arms above her head, with 2/5 strength in both anterior deltoids/bicep brachii/coracobrachialis and 2/5 strength in the psoas major and biceps femoris bilaterally. The patient was subsequently admitted to the hospital for further diagnostic workup.
Diagnosis
Initial laboratory values are shown in Table 1.
 Click to view | Table 1. Initial Laboratory Values on Admission |
A computed tomography (CT) scan of the patient’s abdomen, pelvis, and chest was negative for any malignant process. Magnetic resonance imaging (MRI) of the right and left femur was performed, demonstrating diffuse edema throughout the musculature, consistent with myositis (Fig. 1). A myomarker 3 profile panel was negative for anti-Jo-1 Ab, anti-U1-RNP Ab, anti-SRP Ab, and anti-MI-2 Ab. Anti-HMGCR Ab IgG came back at greater than > 200 units (reference range, < 20). A muscle biopsy of the right vastus lateralis revealed myonecrosis infiltrated by chronic inflammatory cells, consisting of CD68(+) histiocytes/macrophages, CD3(+) T cells, and a small number of CD8(+) cytotoxic T cells. Given the clinical findings of proximal muscle weakness and the laboratory findings of elevated CK and aspartate aminotransferase (AST), myositis was suspected. The differential diagnosis included polymyositis, dermatomyositis, inclusion body myositis, drug-induced myositis, anti-synthetase syndrome, and SINAM. Dermatomyositis was initially the highest on the differential list, given the patient’s heliotrope rash. Inclusion body myositis was considered less likely as there was little involvement of the distal flexor muscles, a characteristic finding. However, once the anti-HMGCR Ab IgG came back positive, a diagnosis of SINAM was established.
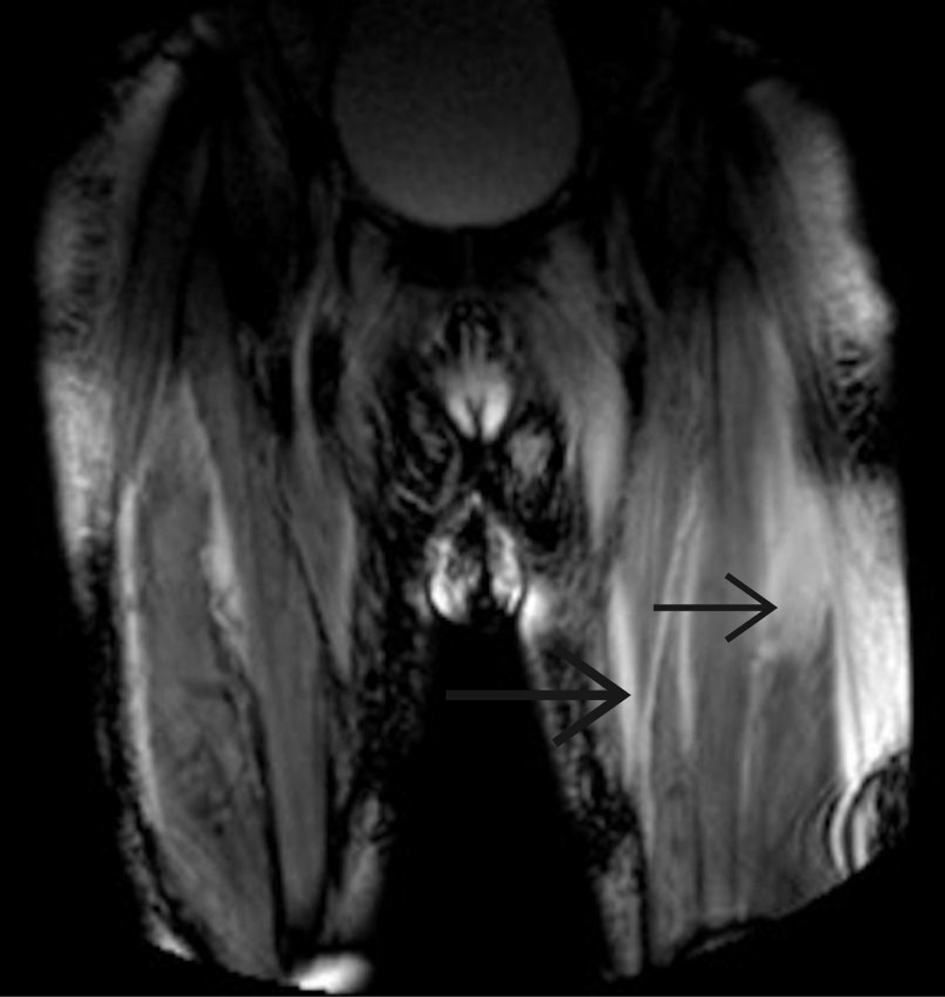 Click for large image | Figure 1. Magnetic resonance imaging of left and right femurs showing diffuse edema consistent with an acute inflammatory myositis. |
Treatment
On the first day of admission, the patient was started on a 3-day course of 1-g intravenous (IV) methylprednisolone. Given that the patient presented with an acute kidney injury (AKI) (her creatinine was 2.76 mg/dL on admission, baseline 0.9 mg/dL) likely secondary to rhabdomyolysis, the plan was to initiate IV immunoglobulin (IVIG) therapy only after her renal function improved. All IVIG products carry an increased risk of renal dysfunction. On day 4, her renal function significantly improved, and she began a 4-day course of 0.4 g/kg/day IVIG therapy; an official diagnosis of statin-induced necrotizing myositis was made. On day 7, the patient was started on a regimen of mycophenolate mofetil, beginning with 500 mg twice daily and increasing the dosage every 2 days until a dose of 1,500 mg twice daily was achieved. On day 13, tacrolimus 2 mg twice daily was added to her maintenance regimen. On day 14, she was deemed stable for discharge on a regimen of mycophenolate mofetil 1,500 mg twice daily and tacrolimus 2 mg daily. Throughout the hospital course, the patient’s CK down trended, her creatinine normalized, and she regained some of her strength.
Follow-up and outcomes
Two months later, she continues to follow up with the rheumatology clinic and has regained most of her strength. She continues to take mycophenolate 1,500 mg twice daily and tacrolimus 2 mg daily.
| Discussion | ▴Top |
Statins, one of the most widely prescribed medications, are potent cholesterol-lower medications. They work by inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA), an enzyme that catalyzes the conversion between HMG-CoA and mevalonic acid. Downregulation of intrahepatic cholesterol pools upregulates hepatic low-density lipoprotein (LDL) receptors, thus decreasing LDL cholesterol/LDL particle levels within the bloodstream.
Statin myopathy, the most common adverse reaction to statins, is estimated to affect anywhere from 6-10% of patients on statin therapy [2]. Although the precise mechanism of this adverse reaction has not been fully elucidated, some studies suggest that statins decrease coenzyme q10 levels in the serum, which may impair mitochondrial function within the muscle [3]. Coenzyme q10 supplementation is a popular therapy for myalgia. Despite this posited mechanism, there is conflicting evidence for supplementation. In some patients, statin myopathy can be accompanied by mild elevation of CK (< 1,000 IU/L), which in itself is not clinically dangerous. On the same spectrum of disease is a statin-related self-limited toxic myopathy, a self-limiting disease course that warrants dose reduction or discontinuation of the statin [4, 5].
Very rare and potentially life-threatening, SINAM is an autoimmune phenomenon that has been reported to affect 2 - 3 in 100,000 patients that take statins [4]. Risks for developing this disease increase with age, and it disproportionately affects women [4]. Patients present with acute onset proximal muscle weakness and CK levels 10 - 100 times of the normal limit (2,000 - 20,000 IU/L). It has been suggested that atorvastatin, simvastatin, and lovastatin (all three of which are oxidized by the cytochrome p450 3A4) are associated with higher rates of adverse effects, as compared to pravastatin and fluvastatin (not oxidized by cytochrome p450 3A4) [6]. Detection of anti-HMGCR antibodies is necessary to confirm the diagnosis of SINAM.
The pathogenesis of this disease has yet to be elucidated. HMG-CoA reductase, the enzyme that statins inhibit, is constitutively expressed by muscle cells at low concentrations [2]. Statins upregulate the expression of HMG-CoA reductase. When increased in concentration, it is hypothesized that this enzyme can effectively act as an autoantigen and induce the production of anti-HMGCR antibodies [5]. It has been suggested that the interaction between statins and HMG-CoA reductase may induce structural and confirmational changes within the protein, generating epitopes to which the immune system can react [4].
Pathological findings on muscle biopsy show necrosis of muscle fibers with a macrophage infiltration [4-10]. Lymphocytic infiltrate is more limited compared to other myosotis and is predominated by macrophages. Whether anti-HMGCR antibodies are causally responsible for myocyte injury is not fully understood. However, it has been observed that these antibody levels are correlated with CK levels [4].
Genetic studies have found a strong association between the HLA class II allele DRB1*11:01 and the development of anti-HMG-CoA reductase autoantibodies, with odds ratios ranging from 25 to 57 [4, 7, 11, 12]. However, given that not all patients on statin therapy with this allele develop SINAM, the etiology of this disease is likely multifactorial.
Treatment of SINAM includes the discontinuation of statin and initiation of immunosuppressive therapy. It is important to note that there have been no clinical trials for the treatment of SINAM. Therefore, limited guidance exists beyond case studies to help clinicians navigate therapeutic strategies. Most clinicians will rely on their prior experience in treating other autoimmune diseases. Initial immunosuppressive therapy includes high-dose corticosteroid therapy with or without IVIG therapy. It is recommended that shortly after the initiation of steroid therapy, agents such as azathioprine, methotrexate, and mycophenolate mofetil, should be initiated [4, 13]. Therapeutic response is monitored by serum CK levels and reported symptoms. If the patient’s symptoms do not improve with a combination of these therapies, rituximab may be added. In nearly 50% of patients diagnosed with SINAM, treatment with steroids, an immunosuppressive agent, and IVIG/rituximab is required [4]. IVIG alone is rarely sufficient, though it has been reported [14].
Maintenance therapy with methotrexate, mycophenolate mofetil, or azathioprine is usually required until the patient recovers full strength. These medications should eventually be tapered, closely monitoring the patient’s symptoms. It is advised that clinicians monitor both muscle strength and CK levels, though it has been reported that CK levels remain elevated despite muscle strength recovery. It should be noted that in the context of muscle atrophy/fatty displacement of muscle tissue, patients may not recover full strength [4].
Conclusion
SINAM is a rare and deadly manifestation of statin myalgia. With an increase in cases being reported, the pathogenesis must be elucidated. With an increase in our understanding of its pathogenesis, one day, we hope to identify patients susceptible to this life-threatening disease and reduce morbidity and mortality.
Learning points
Statin myalgias exist on a broad spectrum, ranging from benign myalgias with or without elevated CK levels to SINAM, a dangerous and life-threatening disease. While a heliotrope rash is commonly associated with dermatomyositis, it can be non-specific and seen in SINAM. Confirmation is made with anti-HMGCR antibodies. We report our experience with SINAM, given its rarity and limited guidance on treatment. We hope our success will guide physicians encountering this disease.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written consent was obtained.
Author Contributions
All authors contributed to the writing/editing part of this paper. Yonatan Akivis wrote the manuscript, Meenakshi Kurup edited the manuscript, and Sabu John edited and guided us in writing this manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CK: creatinine kinase; Anti-HMG-CoA: anti-3-hydroxy-3-methylglutaryl-coenzyme A; SINAM: statin-induced necrotizing autoimmune myositis; IVIG: intravenous immunoglobulin
| References | ▴Top |
- Bytyci I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, Thompson PD, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43(34):3213-3223.
doi pubmed - Hamann PD, Cooper RG, McHugh NJ, Chinoy H. Statin-induced necrotizing myositis - a discrete autoimmune entity within the "statin-induced myopathy spectrum". Autoimmun Rev. 2013;12(12):1177-1181.
doi pubmed - Ruano G, Windemuth A, Wu AH, Kane JP, Malloy MJ, Pullinger CR, Kocherla M, et al. Mechanisms of statin-induced myalgia assessed by physiogenomic associations. Atherosclerosis. 2011;218(2):451-456.
doi pubmed - Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374(7):664-669.
doi pubmed - Gawey B, Tannu M, Rim J, Sperling L, Henry TL. Statin-induced necrotizing autoimmune myopathy. JACC Case Rep. 2020;2(3):440-443.
doi pubmed - Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C-60C.
doi pubmed - Selva-O'Callaghan A, Alvarado-Cardenas M, Pinal-Fernandez I, Trallero-Araguas E, Milisenda JC, Martinez MA, Marin A, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215-224.
doi pubmed - Nazir S, Lohani S, Tachamo N, Poudel D, Donato A. Statin-associated autoimmune myopathy: a systematic review of 100 cases. J Clin Rheumatol. 2017;23(3):149-154.
doi pubmed - Stenzel W, Goebel HH, Aronica E. Review: immune-mediated necrotizing myopathies—a heterogeneous group of diseases with specific myopathological features. Neuropathol Appl Neurobiol. 2012;38(7):632-646.
doi pubmed - Alshehri A, Choksi R, Bucelli R, Pestronk A. Myopathy with anti-HMGCR antibodies: Perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e124.
doi pubmed - Mammen AL, Gaudet D, Brisson D, Christopher-Stine L, Lloyd TE, Leffell MS, Zachary AA. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res (Hoboken). 2012;64(8):1233-1237.
doi pubmed - Patel J, Superko HR, Martin SS, Blumenthal RS, Christopher-Stine L. Genetic and immunologic susceptibility to statin-related myopathy. Atherosclerosis. 2015;240(1):260-271.
doi pubmed - Chung T, Christopher-Stine L, Paik JJ, Corse A, Mammen AL. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve. 2015;52(2):189-195.
doi pubmed - Mammen AL, Tiniakou E. Intravenous Immune Globulin for Statin-Triggered Autoimmune Myopathy. N Engl J Med. 2015;373(17):1680-1682.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


