| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 1, January 2023, pages 31-35
A Presentation of Synchronous Ovarian and Endometrial Endometrioid Adenocarcinoma From a Case of Suspected Ruptured Ectopic Pregnancy
Siak Ming Goha, c, Yanlin Carly Wub, Ryan Wai Kheong Leea
aDepartment of Minimally invasive Surgery Unit, Division of Obstetrics and Gynaecology, KK Women’s and Children’s Hospital, 100 Bukit Timah Road, Singapore 229899, Singapore
bDepartment of Obstetrics and Gynaecology, National University Hospital, Singapore 119074, Singapore
cCorresponding Author: Siak Ming Goh, Department of Minimally invasive Surgery Unit, Division of Obstetrics and Gynaecology, KK Women’s and Children’s Hospital, 100 Bukit Timah Road, Singapore 229899, Singapore
Manuscript submitted December 9, 2022, accepted January 10, 2023, published online January 18, 2023
Short title: Synchronous Ovarian and Endometrial Malignancy
doi: https://doi.org/10.14740/jmc4011
| Abstract | ▴Top |
A 32-year-old woman of child-bearing age who initially underwent surgical laparoscopy for suspected ruptured ectopic pregnancy with elevated serum β-human chorionic gonadotropin (hCG) levels was unexpectedly found to have histologically diagnosed synchronous ovarian and endometrial endometrioid adenocarcinoma. She subsequently underwent another full completion staging surgery and adjuvant chemotherapy as she was unsuitable for fertility-sparing surgery. An elevated serum β-hCG level accompanied by clinical signs of acute abdominal pain, per vaginal bleeding, ultrasound features of abdominal free fluid in the pelvis and an adnexal mass warrants a high clinical suspicion for a ruptured ectopic pregnancy. However, it is important to recognize ovarian malignancy as a rare but differential diagnosis to suspected ectopic pregnancy in patients with acute abdomen. Fertility-sparing surgery may be considered for young patients seeking fertility, without compromising patient survival in women without synchronous gynecological cancers.
Keywords: Synchronous; Ovarian; Endometrial; Adenocarcinoma; Ruptured; Ectopic; Pregnancy
| Introduction | ▴Top |
An elevated serum β-human chorionic gonadotropin (hCG) level accompanied by clinical signs of acute abdominal pain, per vaginal bleeding, ultrasound features of abdominal free fluid in the pelvis and an adnexal mass warrants a high clinical suspicion for a ruptured ectopic pregnancy, requiring urgent surgical intervention. We present a case of a 32-year-old woman of child-bearing age who initially underwent surgical laparoscopy for suspected ruptured ectopic pregnancy with elevated serum β-hCG levels but was unexpectedly diagnosed with synchronous ovarian and endometrial endometrioid adenocarcinoma histologically.
Endometrioid adenocarcinomas of the ovary are malignant epithelial tumors associated with endometriosis [1]. Up to one-third of patients with endometrioid carcinomas of the ovary also have synchronous endometrial hyperplasia or endometrial carcinomas [2] with significant implications on fertility. She subsequently underwent another full completion staging surgery and adjuvant chemotherapy as she was unsuitable for fertility-sparing surgery given dual synchronous gynecological cancers. Fertility-sparing surgery may only be considered in a selected group of young patients seeking fertility, without compromising patient survival [3].
| Case Report | ▴Top |
Investigations
A 32-year-old nulliparous woman with no significant past medical history was referred to the Urgent Obstetrics and Gynecology Center where she presented with a sudden onset of severe lower abdominal pain associated with vomiting for 2 days on a background of per vaginal spotting for 5 days.
On arrival, her vital signs showed tachycardia of 121 beats per minutes with a normal temperature of 37 °C and blood pressure of 101/87 mm Hg. On examination, her abdomen was soft but distended with generalized tenderness. There was no rebound tenderness or guarding. Murphy’s sign was negative. Speculum examination showed a normal appearing cervix with cervical os closed and a small amount of old blood in the vagina. A bedside ultrasound scan showed an empty uterus, a large amount of free fluid in the abdomen and pelvis and a left adnexal mass. (Fig. 1a, b). Initial investigations revealed a positive urine pregnancy test with slightly elevated serum β-hCG of 8 IU/L, hemoglobin of 9.9 g/dL, white blood cell count of 38.5 × 103/µL and markedly raised creatinine of 176 µmol/L and urea of 9.2 mmol/L. Liver function test, serum amylase and lipase were normal.
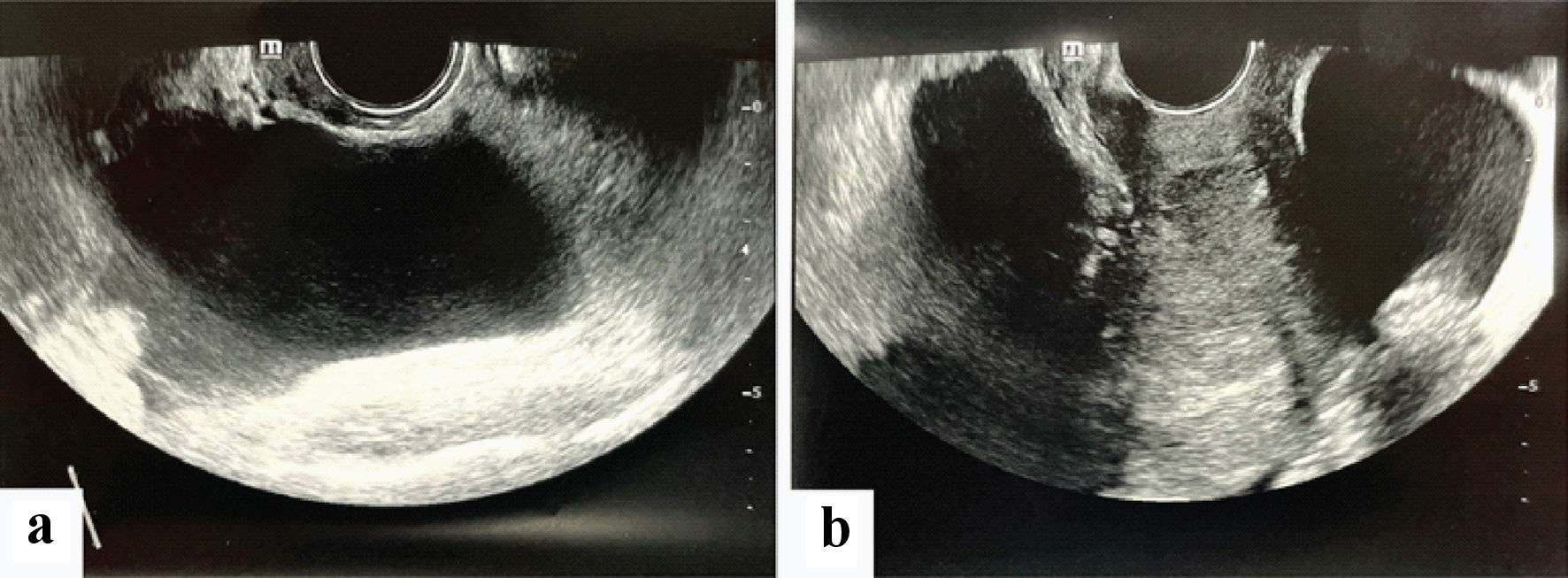 Click for large image | Figure 1. (a, b) Transvaginal ultrasound images showing a large cystic mass with surrounding free fluid. |
Diagnosis
The clinical diagnosis then was that of a suspected ruptured ectopic pregnancy. The patient was counseled on differential diagnosis of an ovarian mass and the possibility of negative laparoscopy for ectopic pregnancy. The patient was agreeable for and underwent an emergency diagnostic laparoscopy keep in view salpingectomy keep in view proceed.
Treatment
Intraoperative findings revealed large amounts of ascitic fluid in the abdominal cavity. A total of 2,550 mL of ascitic fluid was aspirated. There was no hemoperitoneum seen in the pelvis. Peritoneal survey performed showed a large 20-cm multiloculated large ovarian mass in the pelvis arising from the left ovary with small bowel adhesions to the mass (Fig. 2a-d). The uterus, left tube, right tube and ovary appeared normal and no fallopian ectopic mass was seen.
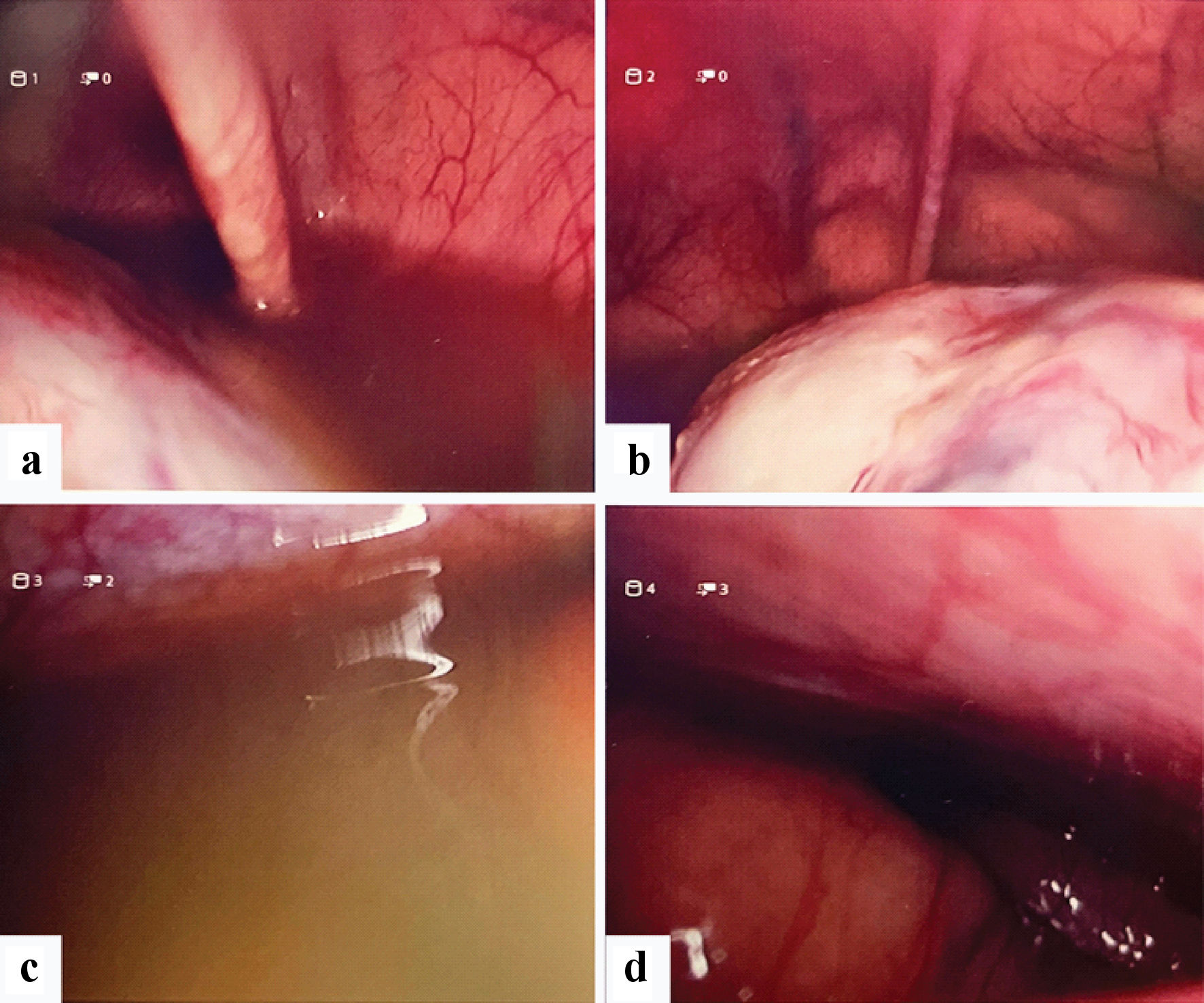 Click for large image | Figure 2. Laparoscopy images. (a) Free fluid in pelvis, pelvic mass. (b) Pelvic mass. (c) Gross ascites. (d) Liver appears normal. |
Clinical decision was made to convert to a midline laparotomy instead in view of the intraoperative findings above. Adhesiolysis was performed to free the cyst from the small bowel and a left salpingo-oophorectomy was performed. The ovarian mass was removed whole and intact (Fig. 3a, b). A small bowel serosal defect was repaired. Hemostasis was ensured and the abdomen was closed in layers. A left pelvic drained was placed. Gentle curettage to the endometrium did not yield any obvious products of conception. The estimated blood loss was 300 mL.
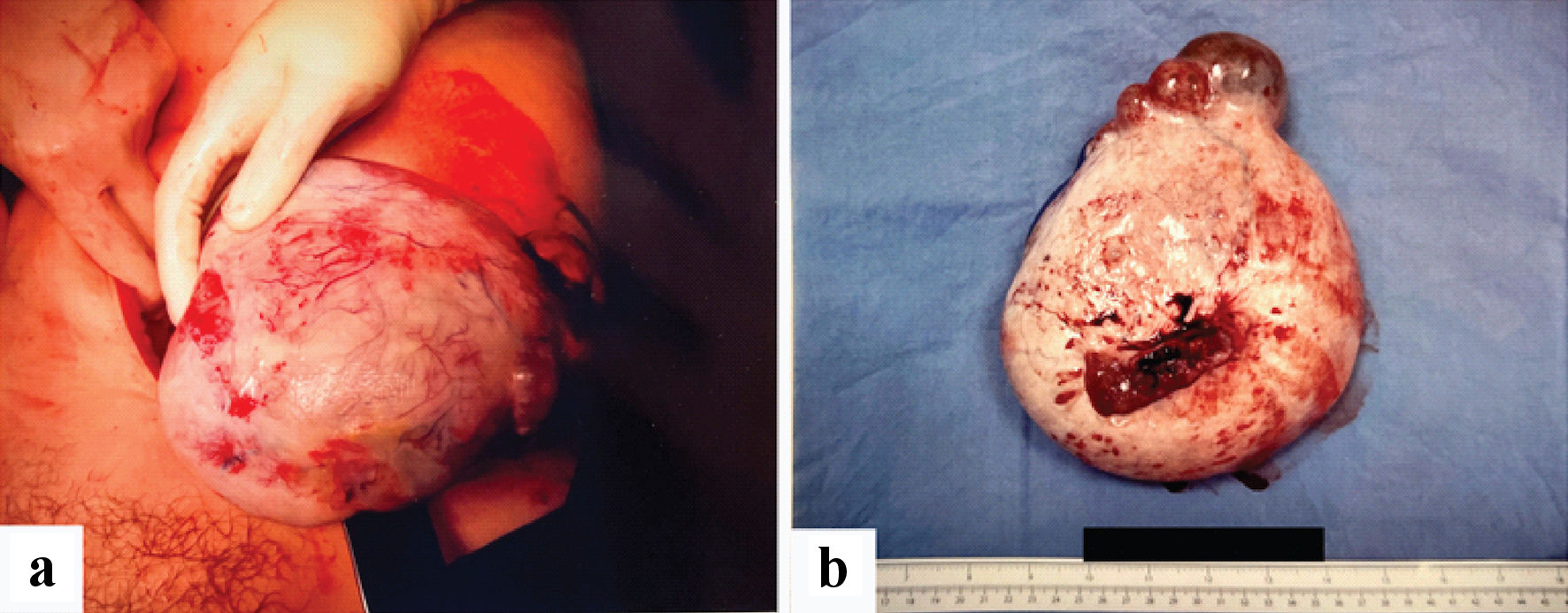 Click for large image | Figure 3. (a, b) Specimen images of large left ovarian mass. |
Postoperatively, the patient was monitored in the intensive care unit for acute kidney injury and T-wave inversion noted intraoperatively. Serum creatinine later normalized to 78 µmol/L 12 h post operation, and serum troponin down-trended from 122 ng/L to 54 ng/L. The patient was then transferred to high dependency ward on postoperative day 1, and then to general ward on postoperative day 2. The pelvic drain output down-trended and was removed on postoperative day 4. The patient was discharged well on that same day.
Further investigations postoperatively included ovarian tumor markers, which revealed raised carcinoembryonic antigen (CEA) of 40.5 µg/L and a markedly raised cancer antigen (CA) 125 of 3,099.2 kU/L. A computed tomography of the thorax, abdomen and pelvis was done for staging, which showed no evidence of residual tumor at the surgical bed, no regional or systemic lymphadenopathy and no evidence of metastatic disease.
Histopathological analysis of the operative specimen showed a well differentiated endometrioid cystadenocarcinoma arising in endometriotic cysts. There was no vascular space tumor emboli or surface involvement, and the left fallopian tube was normal. The ascitic fluid was found to contain hyperplastic mesothelial cells and was negative for malignant cells.
Follow-up and outcomes
Subsequently, the patient was later referred to the gynecological oncology service for discussion of fertility-sparing surgery as she was a young 32-year-old nulliparous female with desire for future fertility. Her case was discussed at a multidisciplinary meeting with a panel of specialists including gynecological oncology, medical oncology, radiation oncology, pathology, and radiology. The patient was counseled on the options of full ovarian cancer staging surgery versus fertility-sparing surgery, and the possibility of a third surgery for full ovarian cancer staging if fertility-sparing staging were to yield malignant histology.
The patient opted for fertility-sparing surgery and underwent a fertility-sparing laparoscopic staging surgery with left pelvic lymph node dissection, para-aortic lymph node dissection, infracolic omentectomy, adhesiolysis, right ovarian nodule biopsy, bladder peritoneum biopsy, hysteroscopy dilation and curettage and hydrotubation. Intraoperatively, a bladder peritoneum nodule of 1 cm and a right ovarian nodule of 2 cm were biopsied. The endometrium appeared fluffy on hysteroscopy and curettage was performed. The right fallopian tube was found to be patent on hydrotubation.
Histological examination revealed that the bladder, peritoneal and ovarian nodules were in keeping with a diagnosis of endometrioid carcinoma, the International Federation of Gynecology and Obstetrics (FIGO) grade 1. Pelvic lymph nodes obtained were benign. Endometrial curettings were also in keeping with endometrioid endometrial carcinoma, FIGO grade 1.
As histology showed synchronous ovarian and endometrial endometrioid adenocarcinoma, the patient was counseled that she was unfortunately not suitable for fertility-sparing surgery. She finally underwent completion full cancer staging surgery with total laparoscopic hysterectomy, right salpingo-oophorectomy, right pelvic lymph node dissection and peritoneal biopsies. Histological examination revealed endometrial endometrioid carcinoma of 20 mm involving the lower uterine segment, with 11% myometrial invasion. The isthmus was involved while cervical glands and stroma were not involved. There was no lympho-vascular invasion. A focus of grade 1 endometrioid carcinoma of the right ovary arising in an endometriotic cyst was also noted with no ovarian surface involvement. Peritoneal biopsies and dissected pelvic lymph nodes did not show any evidence of malignancy. Peritoneal washing cytology, however, was suspicious for adenocarcinoma.
Consequently, the patient was finally diagnosed to have a grade 1, stage IIB synchronous ovarian and endometrial endometrioid adenocarcinoma after clinical-pathological features favored a more likely dual primary cancer, given her younger age, earlier stage and histologically lower grade as compared to metastatic disease and the tumor did not have any choriocarcinomatous elements. A multidisciplinary tumor board discussion among the various specialists as mentioned previously suggested for postoperative adjuvant chemotherapy which she later received.
| Discussion | ▴Top |
Risk factors for ectopic pregnancy include previous adnexal surgery, pelvic inflammatory disease, use of intra-uterine devices (IUD), endometriosis, and previous fertility treatment [4]. Other differential diagnoses for ectopic pregnancy also include ovarian masses like endometriomas and ovarian cancer. Correct diagnosis of ectopic pregnancy is made based on patient history, clinical acumen, serum β-hCG levels, pelvic ultrasound with preoperative diagnosis often difficult, challenging and unpredictable in our case where the ovarian mass was “masked” in its similar presentation to an ectopic pregnancy, with large amounts of free fluid in the abdo-pelvis suggestive of hemoperitoneum instead of ascites. Multiple cases of neoplasms mimicking ectopic pregnancies have also been reported in literature, including that of germ cell tumors and mature teratomas, making the diagnosis further challenging [5].
While the median age of diagnosis of endometrioid adenocarcinomas is in the sixth decade [6], it can also arise in women of child-bearing age and mimic an ectopic pregnancy in its acute presentation. The most common presenting symptoms of an endometrioid carcinoma of the ovary are pelvic pain with gastrointestinal symptoms, abdominal distension and per vaginal bleeding, all of which were present in the patient discussed above. The combination of a raised β-hCG and an adnexal mass in a female of reproductive age was highly suggestive of an ectopic pregnancy until proven otherwise. Additional features of free fluid in the abdo-pelvis on ultrasound, acute onset of generalized abdominal pain and a low hemoglobin further warrants the suspicion of a ruptured ectopic pregnancy associated with increased morbidity and mortality that warranted an emergency surgical intervention.
The incidence of synchronous ovarian and endometrial cancer occurs in 3.1-10.0% of patients with endometrial cancer and 10% of those with ovarian cancer [7, 8]. These tumors are more common in younger patients, are associated with low-grade disease and can be diagnosed at an early stage like our patient [9]. The etiology of synchronous ovarian and endometrial cancer is not well understood where several mechanisms have been proposed including the extended or secondary mullerian system and theories of endometriosis involving a malignant transformation on endometriotic implants and environmental, immunological, hormonal and genetic factors [10, 11]. The origins of synchronous ovarian and endometrial cancer include dual primary cancer, endometrial cancer with ovarian metastasis and ovarian cancer with endometrial metastasis [12].
Distinguishing between dual primary cancer from metastatic disease is essential for choosing the optimal adjuvant treatment and predicting patient prognosis. Reported clinical-pathological features of dual primary cancer include younger age, earlier stage, histologically lower grade, and a more favorable prognosis than metastatic disease [13]. When intraoperative findings yielded an ovarian mass with gross ascites rather than an ectopic pregnancy with hemoperitoneum, decision was made to remove the tube and ovary for pathological diagnosis and assess patient’s desire for future fertility. Follow-up fertility-sparing staging surgery was attempted; however, with a diagnosis of synchronous ovarian and endometrial endometrioid adenocarcinoma, the patient eventually required completion ovarian cancer staging surgery with a total laparoscopic hysterectomy, right salpingo-oophorectomy, pelvic lymph node dissection and infracolic omentectomy.
Fertility-sparing surgery is currently safe and feasible in stage IA and IC grade 1 - 2 non-clear cell epithelial ovarian cancer. A multidisciplinary approach may help these patients achieve their fertility goals [14]. However, controversial areas include evaluating the safety of fertility-sparing surgery in patient with high-risk factors such as stage IC, grade 3 tumors, and clear cell histology and type of surgery performed such as salpingo-oophorectomy vs. cystectomy [15]. Patients with synchronous tumors localized to the uterine body and adnexa showed a very low risk for recurrence. Currently, further studies are necessary to identify selected group of patients who may benefit with adjuvant therapy [16].
In conclusion, an elevated serum β-hCG level accompanied by clinical signs of acute abdomen with ultrasound features of abdominal free fluid in the pelvis and an adnexal mass warrants a high clinical suspicion for a ruptured ectopic pregnancy. It is also important to recognize ovarian malignancy as a rare but differential diagnosis to suspected ectopic pregnancy in patients with acute abdomen where urgent surgical intervention is necessary.
Learning points
It is important to consider malignant ovarian neoplasm as a differential diagnosis in patients presenting with raised β-hCG level and an adnexal mass. Furthermore, it is paramount to evaluate a patient’s presentation and clinical condition as a whole and intervene emergently when an immediate life-threatening condition such as ruptured ectopic pregnancy is suspected. When a diagnosis of endometrioid adenocarcinoma is made in a young female of reproductive age with desire for future fertility, urgent timely referral to a gynecologic oncology service is essential for assessing suitability for fertility-sparing treatment and follow-up. Up to one-third of patients with endometrioid carcinomas of the ovary also have synchronous endometrial hyperplasia or endometrial carcinomas with significant implications on fertility.
While the gold standard treatment for ovarian cancer remains as full completion staging surgery, a multidisciplinary tumor board discussion for fertility-sparing surgery in women of child-bearing age with early-stage disease may be considered in suitable women and definitive treatment should be pursued without delay.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent has been obtained for this paper.
Author Contributions
All authors contributed equally to the writing of this case report. Siak Ming Goh wrote up the introduction and case report, Yanlin Carly Wu and Ryan Wai Kheong Lee both wrote up the discussion of this manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Farolfi A, Altavilla A, Morandi L, Capelli L, Chiadini E, Prisinzano G, Gurioli G, et al. Endometrioid cancer associated with endometriosis: from the seed and soil theory to clinical practice. Front Oncol. 2022;12:859510.
doi pubmed - Rasuli B, Stanislavsky A. Endometrioid carcinoma of the ovary. In: Radiopaedia.org [Internet]. Radiopaedia.org; 2011 [cited Aug 17, 2022]. Available from: http://radiopaedia.org/articles/13420.
doi - Jiang X, Yang J, Yu M, Xie W, Cao D, Wu M, Pan L, et al. Oncofertility in patients with stage I epithelial ovarian cancer: fertility-sparing surgery in young women of reproductive age. World J Surg Oncol. 2017;15(1):154.
doi pubmed - Parashi S, Moukhah S, Ashrafi M. Main risk factors for ectopic pregnancy: a case-control study in a sample of Iranian women. Int J Fertil Steril. 2014;8(2):147-154.
- Kucera C, Cox-Bauer C, Miller C. Apparent ectopic pregnancy with unexpected finding of a germ cell tumor: A case report. Gynecol Oncol Rep. 2017;21:31-33.
doi pubmed - Nucci MR, Parra-Herran C. Metastatic and miscellaneous primary neoplasms of the ovary. In: Gynecologic Pathology. Elsevier; 2020. p. 749-827. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780323359092000175.
doi - Song T, Seong SJ, Bae DS, Suh DH, Kim DY, Lee KH, Lim MC, et al. Synchronous primary cancers of the endometrium and ovary in young women: a Korean Gynecologic Oncology Group Study. Gynecol Oncol. 2013;131(3):624-628.
doi pubmed - AlHilli MM, Dowdy SC, Weaver AL, St Sauver JL, Keeney GL, Mariani A, Podratz KC, et al. Incidence and factors associated with synchronous ovarian and endometrial cancer: a population-based case-control study. Gynecol Oncol. 2012;125(1):109-113.
doi pubmed - Melin A, Lundholm C, Malki N, Swahn ML, Sparen P, Bergqvist A. Endometriosis as a prognostic factor for cancer survival. Int J Cancer. 2011;129(4):948-955.
doi pubmed - Lauchlan SC. The secondary Mullerian system. Obstet Gynecol Surv. 1972;27(3):133-146.
doi pubmed - Varma R, Rollason T, Gupta JK, Maher ER. Endometriosis and the neoplastic process. Reproduction. 2004;127(3):293-304.
doi pubmed - Lim YK, Padma R, Foo L, Chia YN, Yam P, Chia J, Khoo-Tan H, et al. Survival outcome of women with synchronous cancers of endometrium and ovary: a 10 year retrospective cohort study. J Gynecol Oncol. 2011;22(4):239-243.
doi pubmed - Williams MG, Bandera EV, Demissie K, Rodriguez-Rodriguez L. Synchronous primary ovarian and endometrial cancers: a population-based assessment of survival. Obstet Gynecol. 2009;113(4):783-789.
doi pubmed - Mandelbaum RS, Klar M, Takiuchi T, Bainvoll L, Matsuzaki S, Paulson RJ, Matsuo K. Fertility-sparing treatment for early-stage epithelial ovarian cancer: Contemporary oncologic, reproductive and endocrinologic perspectives. J Obstet Gynaecol Res. 2020;46(8):1263-1281.
doi pubmed - Bercow A, Nitecki R, Brady PC, Rauh-Hain JA. Outcomes after fertility-sparing surgery for women with ovarian cancer: a systematic review of the literature. J Minim Invasive Gynecol. 2021;28(3):527-536.e521.
doi pubmed - Yoneoka Y, Yoshida H, Ishikawa M, Shimizu H, Uehara T, Murakami T, Kato T. Prognostic factors of synchronous endometrial and ovarian endometrioid carcinoma. J Gynecol Oncol. 2019;30(1):e7.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


