| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 10, October 2022, pages 509-512
Kluri Anomaly: Newly Introduced Pancreatic Duct Anomaly
Safi Khuria, b, Yoram Klugera
aDepartment of General Surgery, HepatoPancreatoBiliary Surgical Unit, Rambam Medical Care Center, Haifa, Israel
bCorresponding Author: Safi Khuri, Department of General Surgery, HepatoPancreatoBiliary Surgical Unit, Rambam Medical Care Center, Haifa 31096, Israel
Manuscript submitted September 20, 2022, accepted October 14, 2022, published online October 31, 2022
Short title: Kluri Anomaly of the Pancreatic Duct
doi: https://doi.org/10.14740/jmc4012
| Abstract | ▴Top |
Anomalies of the pancreas organ, especially congenital anomalies, are very uncommon, and can involve the pancreatic ductal as well as the pancreatic extra-ductal systems. While these anomalies usually present as an incidental radiological finding during adulthood, sometimes, they can present as recurrent upper abdominal pain as a presentation of recurrent episodes of acute idiopathic pancreatitis. Thus, these anomalies should be regarded in the differential diagnosis list, as a cause, for recurrent idiopathic pancreatitis, especially in the adult age group of patients. Multiple different variations, mainly in duct course and configuration of the pancreatic ductal system have been reported. In addition, duplication anomalies and cystic dilatation of the pancreatic duct are well known anomalies as well. A combined anomaly of the aforementioned anomalies is even rarer. Herein, we present the case of a male patient, 47 years old, with recurrent admissions to different hospitals due to upper abdominal pain and episodes of severe idiopathic acute pancreatitis. Imaging tests, mainly abdomino-pelvic computed tomography (CT) scan and magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) showed a dominant duct of Santorini without divisum along with cystic dilation of the proximal portion of the Santorini duct. Being a very rare pancreatic duct anomaly, the patient was treated by a multidisciplinary team (MDT) of physicians, including pancreas surgeons, gastroenterologists and radiologists. A surgical resection in the form of total pancreatectomy with Roux-en-Y gastrointestinal reconstruction was contemplated. Perioperative and postoperative periods were uneventful. The previously mentioned anomaly is unknown in the English literature and is introduced as new anomaly known as “Kluri”.
Keywords: Congenital pancreas anomalies; Pancreatic duct anomalies; Idiopathic pancreatitis; Recurrent severe pancreatitis; Kluri anomaly
| Introduction | ▴Top |
Congenital anomalies may arise in any organ system of the human body, of which the pancreas is a well-known one. Anomalies of the pancreas could involve the pancreatic ductal (major pancreatic duct: Wirsung duct, and minor pancreatic duct: Santorini duct) as well as the extra-ductal system. These anomalies are very uncommon, and its precise incidence rate is yet to be known. Usually, patients with congenital anomalies of the pancreas are asymptomatic, hence the most common presentation is an incidental radiological finding during adulthood period [1]. On the contrary, sometimes, these anomalies can cause recurrent episodes of upper abdominal pain and acute pancreatitis. Therefore, these anomalies should be included in the differential diagnosis as a cause of adult patients with recurrent episodes of idiopathic acute pancreatitis. To a lesser extent, it can present as an unexplained minor gastrointestinal symptom such as recurrent mild upper abdominal pain, nausea and vomiting [2].
Due to the high number of radiological imaging tests that had been used during the last two decades, the incidence rate of pancreas anomalies has increased exponentially. This in turn makes physicians, especially radiologists, to become more familiar with such anomalies [3]. The used imaging tests nowadays are highly sensitive for the recognition of these anomalies and include mainly abdomino-pelvic high-resolution computed tomography (CT) scan, magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) of the abdomen [3]. Although being regarded as a good diagnostic and therapeutic tool for bile and pancreatic ducts diseases, endoscopic retrograde cholangiopancreatography (ERCP) is an invasive procedure which carries a complications risk such as post-ERCP pancreatitis (the most common complication), cholangitis, bleeding, and perforation, with the latter being the least common. Thus, its use for the diagnosis of pancreatic duct anomalies is far less common than the previously mentioned tests.
Pancreatic ductal anatomy can be subjected to multiple variations in course and in configuration [4]. In addition, duplication anomalies, mainly of the main pancreatic duct, as well as cystic dilation of the terminal portion of the Wirsung and Santorini ducts are well known. Combination anomalies of the aforementioned variations are even rarer and up to date are regarded as unknown and never reported.
Herein, a case of 47-year-old male patient, suffered from recurrent episodes of upper abdominal pain due to severe idiopathic pancreatitis is presented. Radiological exams including abdominopelvic CT scan and MRCP revealed an unknown combination anomaly of the pancreatic ducts, with dominant duct of Santorini without divisum and proximal cystic dilation of the Santorini duct. These findings were never reported in the English literature and newly introduced anomaly as “Kluri” anomaly after the names of the authors Kluger and Khuri.
| Case Report | ▴Top |
Investigations
A 47-year-old healthy male patient, presented to our emergency department complaining of severe upper abdominal pain. The pain lasted for 6 h, started instantly and was described as diffuse and radiating to the back. He also suffered from general weakness, nausea, recurrent vomiting, and reduced appetite. The patient denied fever, rigors, or another symptom. His past medical history included diabetes mellitus (DM) type 2, treated by subcutaneous insulin injections and his surgical history was relevant for status post laparoscopic cholecystectomy due to cholelithiasis at age 40 years.
The patient’s background included a history of two admissions during the last year to different peripheral hospitals, due to severe idiopathic necrotizing pancreatitis. During his admissions, patient suffered from severe pancreatitis with multiorgan failure, which necessitates admission to the intensive care unit (ICU) for several weeks of conservative management. Between the two episodes, the patient complained of continuous upper abdominal pain treated by different narcotics. Few months following the second episode, the patient underwent abdominal MRCP which was consistent with pancreas divisum and 17-mm cystic lesion at the pancreatic head. Moreover, an endoscopic ultrasound (EUS) showed cystic dilation of the duct at the pancreatic head up to 17 mm, and a suspicion for main duct intraductal pancreatic mucinous neoplasm (IPMN) was raised. Cyst aspiration and cystic fluid analysis for amylase and carcinoembryonic antigen (CEA) was not done for unknown reasons.
Diagnosis
Vital signs, upon his admission were within normal limits: blood pressure (BP) 123/86 mm Hg, pulse (P) 76 beats/min, respiratory rate (RR) 11/min, temperature (T): 36.8 °C. Abdominal examination was consistent for diffuse upper abdominal tenderness without guarding, as well as without palpable masses. Digital rectal examination was normal. A complete blood count showed increased white blood cells of 12,000 × 109/L, with 12% band neutrophils. Serum amylase (608 U/L), as well as urine amylase (15,596 U/L) levels were highly elevated. Liver function tests (LFTs), kidney function tests (KFTs) and arterial blood gases (ABGs) were within normal limits. An abdominal ultrasonography (US) showed cystic lesion at the pancreatic head without bile duct dilation or choledocholithiasis. The patient was diagnosed with a recurrent episode of acute pancreatitis and admitted to the General Surgery ward for conservative management by nil per os (NPO), rigorous intravenous (IV) fluids and IV painkillers. Following the initiation of conservative management, the patient felt well and resumed oral intake. Revision of the abdominal MRCP revealed unknown combination anomaly of the pancreatic ducts, with Santorini duct dominance without divisum and cystic dilation of the proximal part of the Santorini duct (Fig. 1).
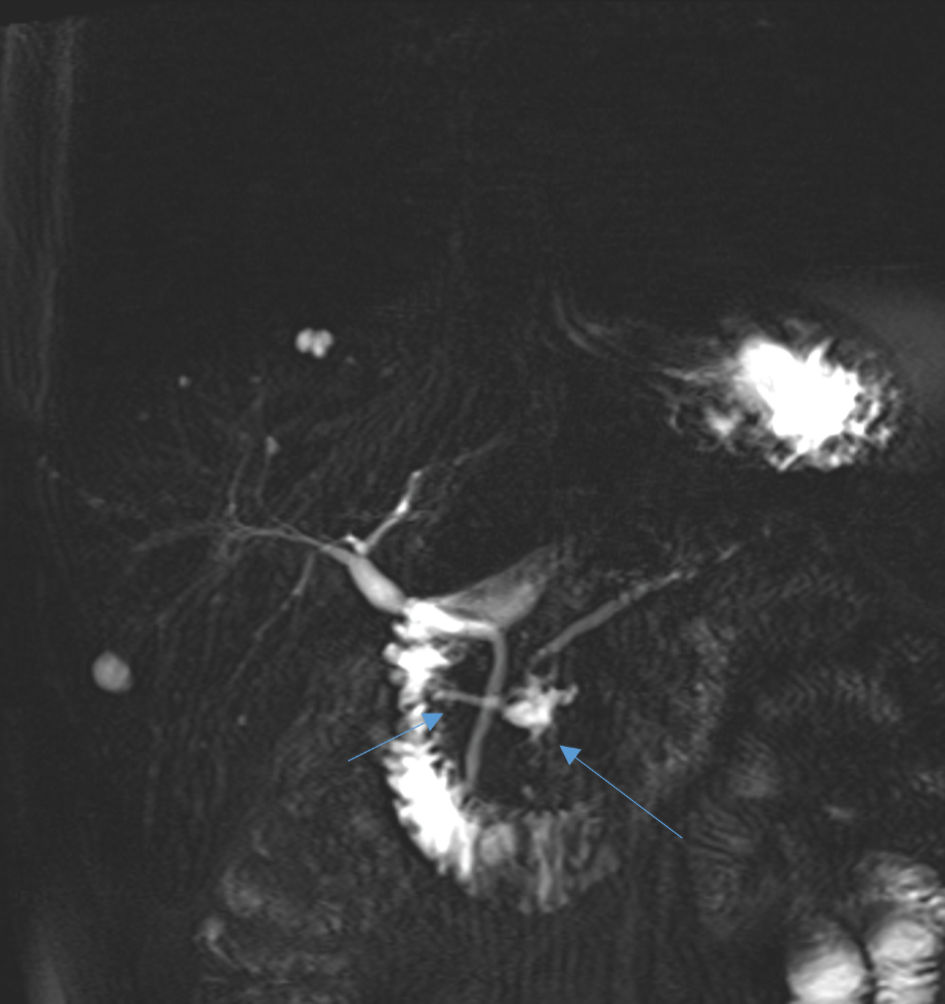 Click for large image | Figure 1. An abdominal MRCP showing dominant duct of Santorini without divisum (short arrow) and proximal cystic dilation of the proximal part of Santorini duct (long arrow). MRCP: magnetic resonance cholangiopancreatography. |
A recent (during current admission) abdomino-pelvic CT scan (Fig. 2) revealed findings identical to what have been seen by the abdominal MRCP; dilation in the form of cyst at the proximal Santorini duct along with dominant duct of Santorini without divisum. In addition, Atrophy of the pancreatic body and tail were demonstrated. Other known pancreatitis causes were excluded: the patient negated alcohol consumption or recent abdomino-thorax trauma. Serum triglycerides (following overnight fasting) and calcium levels were within normal limits. Serum immunoglobulin G (IgG)4 levels (IgG4-related pancreatitis) were as well within normal levels. As there was no clinical suspicion for an infectious agent (hepatitis A, mumps and others) as a causative agent for acute pancreatitis in our patient, no specific laboratory tests were done. As part of our department protocol, tumor markers, mainly CEA and cancer antigen 19-9 (CA19-9) are routine serum tests to be made during the workup program. Serum levels for these tumor markers were within normal limits.
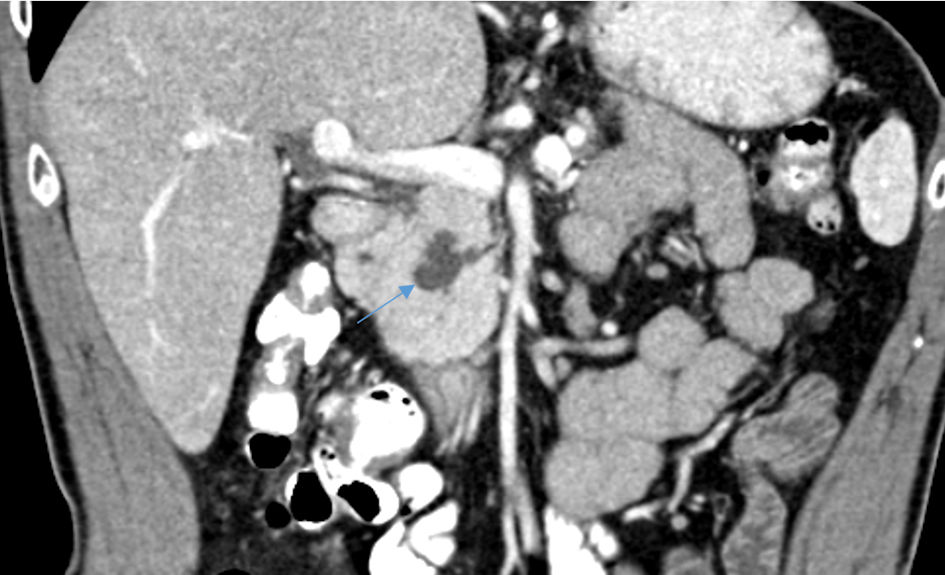 Click for large image | Figure 2. An abdominopelvic CT scan reveals cystic dilation of the proximal part of Santorini duct (arrow). CT: computed tomography. |
Treatment
In light of the aforementioned findings, the patient was presented at a multidisciplinary team meeting (MDTM), including pancreatic surgeons, gastroenterologists and abdominal radiologists. It was decided, that due to the recurrent episodes of severe acute pancreatitis (necessitating admissions to the ICU) in young patient with radiological proven pancreatic duct anomaly and pancreatic body and tail atrophy, surgical resection with total pancreatectomy would be the best approach. Surgical resection in the form of pancreatoduodenectomy (Whipple procedure) was discussed. Due to the fact that the patient is already known to suffer from DM type 2 treated by insulin injections, and if Whipple procedure to be done, most probably the procedure will be without pancreato-jejunostomy anastomosis due to high risk for postoperative pancreatic fistula (atrophy of the pancreatic texture and non-dilated main pancreatic duct), it was decided to resect the whole pancreas organ.
Six weeks after his discharge, the patient was admitted electively to our surgical ward for operative management. On his admission, the patient denied any complaint and was in stable condition. His physical exam was unremarkable. During the patient’s hospitalization he underwent total pancreatectomy with Roux-en-Y reconstruction of the gastrointestinal tract by an open approach surgery. His perioperative and postoperative periods were uneventful. The patient was discharged home on postoperative day 9, and prescribed Creon (pancreatic enzyme supplements) and subcutaneous insulin injections.
Follow-up and outcomes
The patient was followed on our outpatient clinic once per 2 weeks (initially) followed by once/month up to 3 months following discharge. During his follow-up, the patient was doing well with no gastrointestinal or other complaints. The histopathological report was consistent with dilated pancreatic duct at the head, with foci of low-grade Pancreatic Intraepithelial neoplasia (PanIN). Body and tail of the pancreas showed atrophy, fibrosis, and inflammation. No evidence of malignancy was reported.
| Discussion | ▴Top |
Pancreatic duct anomalies are uncommon gastrointestinal congenital diseases, yet a forgotten cause of recurrent idiopathic pancreatitis. Multiple variations of the duct of the pancreas exist and can be at the level of the duct course, duct configuration, duplication anomaly or cystic dilation of the terminal part of the ducts [5]. Combination anomalies of the previously mentioned variations are even rarer.
Although pancreatic duct course can be sigmoidal, vertical or loop, the most common course is the descending one (50%) [1]. Pancreas divisum is known as the most common pancreatic duct anomaly. The pathogenesis for pancreas divisum is as follow: during the initial weeks of gestation, mainly at the seventh gestational week, the dorsal pancreatic bud (which contribute to the formation of the body, tail and minor part of the head of the pancreas, as well as Santorini duct) fails to fuse with ventral pancreatic bud (forms the majority of the pancreatic head and uncinate process) [2, 3]. The exact incidence rate is unknown yet reported rates of 3-8% and almost 9% found on ERCP and MRCP tests, respectively [6, 7]. Ductal configuration anomalies are uncommon. The most common form of pancreatic duct configuration is the bifid configuration with Wirsung dominant duct (60%), followed by absent duct of Santorini (30%), ansa pancreatica and dominant duct of Santorini without pancreas divisum (1%) [4, 5]. Ansa pancreatica is an extremely rare pancreatic duct anomaly and is characterized by reversed S-shaped of the Santorini duct which usually connects the Wirsung duct by a side branch.
A rare pancreatic duct anomalies can also include cystic dilation of the ultimate parts of the different pancreatic ducts. These anomalies are known as Wirsungocele, when it involves the last portion of the Wirsung duct, and Santorinicele, when the terminal Santorini duct is dilated [5]. Cystic dilation of the proximal part of the pancreatic ducts, as well as combination anomalies have never been reported in the English literature. Herein, we present a new and non-reported combination anomaly of the pancreatic duct; proximal cystic dilation of the Santorini duct with dominant Santorini duct without pancreas divisum. As already mentioned before, these findings were never reported in the English literature and are introduced as “Kluri” anomaly after the names of the authors Kluger and Khuri.
Currently, there is not an established scientific-based consensus regarding the best therapeutic strategy to be followed in treating this unique and uncommon pancreatic anomalies. This is largely due to the fact that pancreatic duct anomalies are very rare. Asymptomatic patients found incidentally do not require usually further therapeutic management. On the other hand, the choice of therapy in symptomatic patients depends usually on several parameters such as frequency, severity and duration of symptoms and the presence/absence of complications [8]. Therapeutic intervention (either endoscopic or surgical) is usually indicated for patients with recurrent mild attacks of pancreatitis or following single attack of severe pancreatitis. The importance of multidisciplinary team approach for such cases should be highly appreciated and routinely advocated. Since our patient is young and suffered recurrent episodes of severe acute pancreatitis, MDTM decision was surgical resection in the form of total pancreatectomy with Roux-en-Y reconstruction of the gastrointestinal tract.
In conclusion, congenital pancreatic duct anomalies are rare, yet becoming more familiar medical phenomenon among physicians. In the presence of ductal anomaly along with recurrent episodes of severe pancreatitis, MDTM is highly advisable. Authors are encouraged to report such rare cases to gain knowledge of the different characteristics, presentations, and management of such anomalies.
Learning points
The main take away points from this case report are to know that pancreatic duct anomalies are rare anomalies, and usually forgotten cause of recurrent idiopathic pancreatitis. In addition, due to the recent rise in the use of the highly sensitive abdominal imaging studies for different purposes, physicians are familiar more with these anomalies. In the setting of recurrent idiopathic pancreatitis and proven radiological pancreatic duct anomaly, a MDTM is highly recommended. Herein, we introduce a never reported pancreatic duct anomaly, known as “Kluri” anomaly as a cause of recurrent severe pancreatitis.
Acknowledgments
None to declare.
Financial Disclosure
No funding sources.
Conflict of Interest
The authors declare that they have no competing interest.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author Contributions
SK contributed to writing as well as for the design of the manuscript. YK was the mentor and contributed to critical revisions of the paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CT: computed tomography; MRI: magnetic resonance imaging; MRCP: magnetic resonance cholangiopancreatography; MDT: multidisciplinary team; ERCP: endoscopic retrograde cholangiopancreatography
| References | ▴Top |
- Itoh S, Ikeda M, Ota T, Satake H, Takai K, Ishigaki T. Assessment of the pancreatic and intrapancreatic bile ducts using 0.5-mm collimation and multiplanar reformatted images in multislice CT. Eur Radiol. 2003;13(2):277-285.
doi pubmed - Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointest Endosc Clin N Am. 1995;5(1):1-30.
doi - Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin N Am. 1998;8(1):55-77.
- Mortele KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26(3):715-731.
doi pubmed - Turkvatan A, Erden A, Turkoglu MA, Yener O. Congenital variants and anomalies of the pancreas and pancreatic duct: imaging by magnetic resonance cholangiopancreaticography and multidetector computed tomography. Korean J Radiol. 2013;14(6):905-913.
doi pubmed - Soto JA, Lucey BC, Stuhlfaut JW. Pancreas divisum: depiction with multi-detector row CT. Radiology. 2005;235(2):503-508.
doi pubmed - Morgan DE, Logan K, Baron TH, Koehler RE, Smith JK. Pancreas divisum: implications for diagnostic and therapeutic pancreatography. AJR Am J Roentgenol. 1999;173(1):193-198.
doi pubmed - Gutta A, Fogel E, Sherman S. Identification and management of pancreas divisum. Expert Rev Gastroenterol Hepatol. 2019;13(11):1089-1105.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


