| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 11, November 2022, pages 536-540
A Rare and Unusual Cause of Ischemic Stroke to Be Aware of
Anmol Johala, b, Steven Imburgioa, Viraaj Pannua, Helen Pozdniakovaa, Ndausung Udongwoa, Mohammad Hossaina, Swapnil Patela
aDepartment of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
bCorresponding Author: Anmol Johal, Department of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
Manuscript submitted September 23, 2022, accepted November 7, 2022, published online November 27, 2022
Short title: Ischemic Stroke With Right-Sided ICA Agenesis
doi: https://doi.org/10.14740/jmc4015
| Abstract | ▴Top |
Congenital absence of an internal carotid artery (ICA) is an extremely rare vascular anomaly. This case report presents an instance of right ICA agenesis to highlight the importance of early identification of this anomaly and its impact on disease presentation and complications. With transient ischemic attack (TIA), cerebrovascular accident (CVA), and cerebral aneurysms being among the chief presenting scenarios or course of the anomaly, it is important to have a high level of suspicion for these in patients with known ICA agenesis. Understanding the underlying development of this vasculature and its impact on cerebral circulation aids in identifying possible findings on imaging. This case report aims to delineate the pathophysiology of ICA agenesis, recognition of the vasculature that contributes to the anomaly, different presentations of the disease, complications, and obstacles in management.
Keywords: Internal carotid artery; Agenesis; Ischemic stroke; Cerebral aneurysm; Carotid artery aplasia; Cerebrovascular accident
| Introduction | ▴Top |
Adequate circulation of oxygenated blood is crucial for maintaining proper perfusion to vital organs. The head and neck region receives blood flow from the carotid and vertebral arteries, with a majority of the blood flow to the brain being directly supplied from the two common carotid arteries (CCAs) [1]. The left CCA originates from the arch of the aorta in the thorax, while the right CCA originates from the brachiocephalic artery at the level of the neck [2]. As they ascend, each CCA bifurcates at the level of the carotid sinus into the internal carotid artery (ICA) which supplies the brain, and the external carotid artery (ECA) which supplies structures such as the face and neck [2]. As the primary vehicle for providing oxygen to the brain, the ICAs are one of the most clinically important components of the vascular system.
The clinical significance of the ICA primarily includes carotid artery stenosis as these vessels have a high susceptibility to atherosclerosis [3]. Prolonged plaque buildup can cause critical stenosis leading to transient ischemic attack (TIA) or predisposing individuals to embolic events such as an ischemic stroke if part of the plaque dislodges and travels distally [4]. While less common, carotid artery pathology can also involve conditions including dissection, fibromuscular dysplasia, or extracranial aneurysm [5-7]. Even less reported, however, is the complete congenital absence of one of the internal carotid arteries. The first documented case of ICA agenesis was discovered back in 1787 by Tode on postmortem examination [8]. To date, there have been just over 100 cases of congenital ICA absence reported in the literature [9]. The expected prevalence of this condition is extremely rare with Ryan et al reviewing over 5,000 cerebral magnetic resonance imaging (MRI) and angiograms and only identifying seven patients (0.13%) with either complete agenesis or hypoplasia [9, 10]. Here, we describe an extremely rare case of right-sided ICA agenesis that was found unexpectedly during the diagnostic workup of an acute ischemic stroke.
| Case Report | ▴Top |
Investigations
A 45-year-old male, with a past medical history significant for previous cerebrovascular accident (CVA), hyperlipidemia, hypertension, type 2 bipolar disorder, prior deep vein thrombosis on apixaban, obesity and schizophrenia presented to the emergency department (ED) with neurologic changes of weakness. The patient reported new-onset left-sided weakness, left-hand tingling sensation, and left eye blurriness that started early in the morning. He stated that since the weakness started it had been getting progressively worse. Patient denied any headaches, dizziness, syncope, seizures, dysphagia, memory impairment, chest pain, palpitations, shortness of breath or lower extremity pain. He was sent to the ED by his facility but endorsed that his symptoms resolved prior to arrival. Patient also endorsed prior CVA and residual right lower extremity weakness. He was non-adherent to his medications and stated that he missed a few doses of his aspirin and apixaban but endorsed taking them the morning prior to his admission.
The patient used a cane to aid with mobility. He denied any use of tobacco or illicit drug use in the past, and only drank alcohol occasionally. Family history was non-contributory. His daily home medications were aspirin 81 mg, apixaban 5 mg twice daily, divalproex 500 mg, lisinopril 10 mg, lurasidone 40 mg, metoprolol succinate 100 mg, and simvastatin 20 mg. Initial vital signs were blood pressure of 172/102 mm Hg, heart rate of 94 beats per minute, respiratory rate of 22 breaths per minute, oxygen saturation of 95% on room air, and temperature of 97.5 °F. The cardiopulmonary examination was unremarkable. He was alert and oriented to name, place, and time. Cranial nerves II - XII were all intact. Strength was 5/5 in all upper and lower extremities. Sensations were intact and his gait was normal. The National Institutes of Health Stroke Scale (NIHSS) score was zero. Complete blood count and comprehensive metabolic panel were all within normal reference ranges.
Diagnosis
Due to concern for an acute stroke or TIA, a computed tomography (CT) scan imaging of the head without contrast was performed and the results revealed multifocal areas of encephalomalacia and gliosis within the left parietal/cerebellar lobes, as well as the right occipital lobe, and tortuosity of the basilar artery (Fig. 1). He was unable to tolerate MRI due to claustrophobia. A CT angiogram of the head and neck revealed non-visualization of the right ICA, suggestive of congenital absence of this vessel (Fig. 2). Additionally, right anterior circulation appeared to be supplied by collateral branches of the distal basilar artery and imaging revealed no carotid artery stenosis.
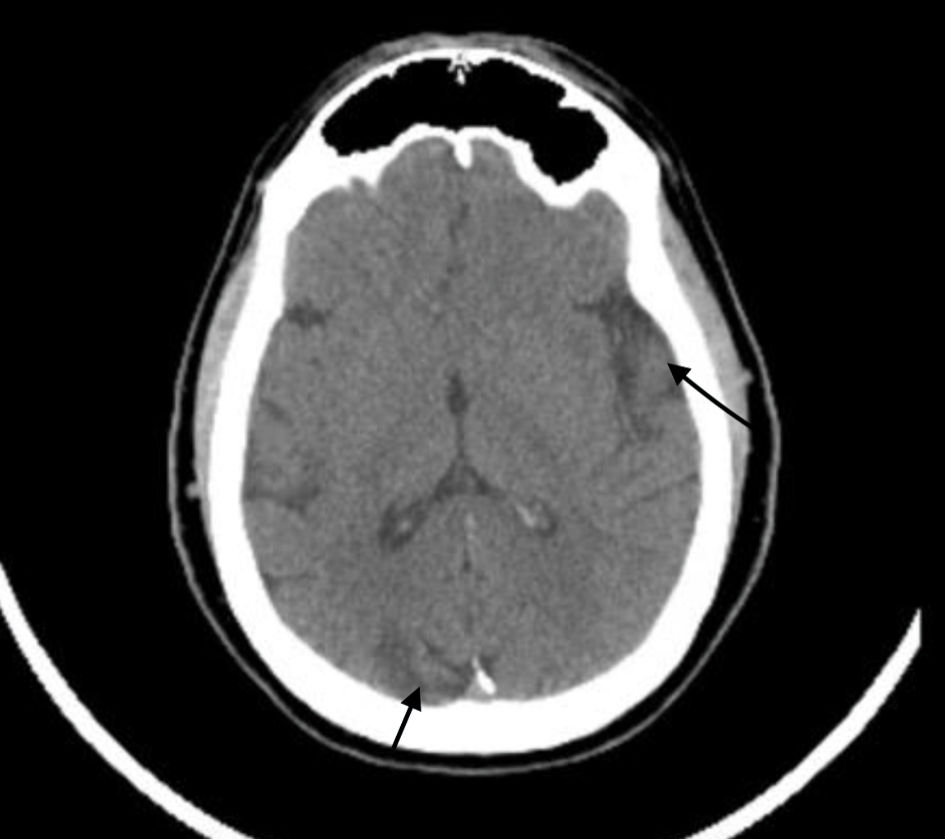 Click for large image | Figure 1. Computed tomography of the head without contrast showing multifocal areas of encephalomalacia and gliosis within the left parietal lobe (long black arrow on right), right occipital lobe (short black arrow at bottom) and left cerebellum. No signs of hemorrhage were noted. |
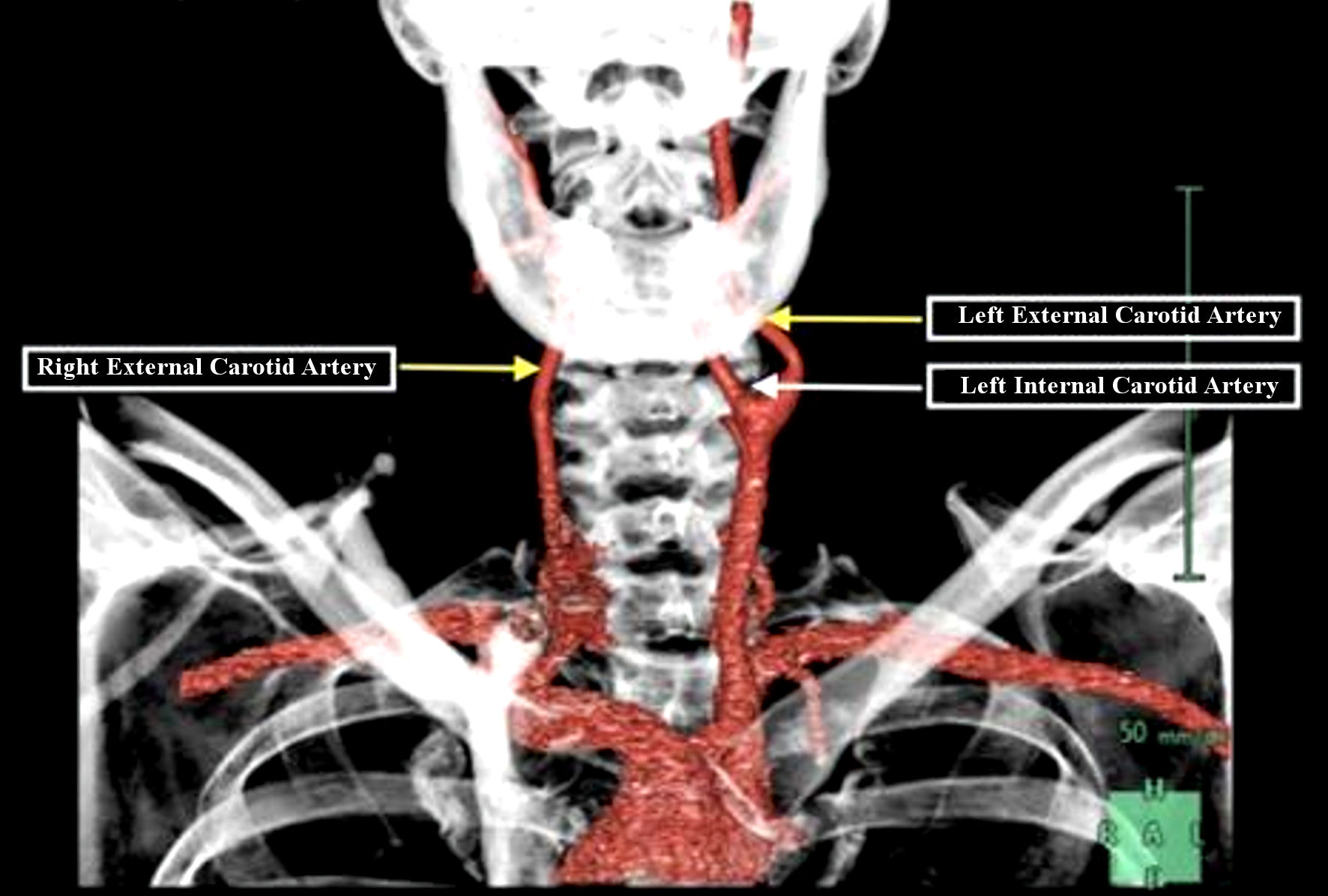 Click for large image | Figure 2. Computed tomography angiogram of the head and neck showing left external (yellow arrow) and internal carotid artery (white arrow) with an isolated right external (yellow arrow) carotid artery with the absence of right internal carotid branch. |
Additional workup for stroke etiology was completed which involved an unremarkable electrocardiogram. A transthoracic echocardiogram revealed an ejection fraction of 55-60% with no left atrial thrombus. The patient was seen by the neurology team and after a comprehensive workup that was negative for other causes of the patient’s ischemic stroke, it was concluded that the patient’s absent right ICA was the underlying etiology. It was believed that his right-sided ICA absence put a strain on his collateral circulation resulting in primarily left-sided sensory and motor symptoms.
Treatment
The patient was already taking aspirin 81 mg and apixaban at home on presentation, so this therapy was continued to hopefully prevent further events. Carotid endarterectomy or endovascular stenting was not considered due to the lack of stenosis in the carotid artery. Patient received close medical and neurological follow-up with regular neuro checks during his stay with no other adverse events occurring.
Follow-up and outcomes
Fortunately, his symptoms fully resolved, patient was able to return to his baseline status and he was discharged on aspirin, apixaban, metoprolol, lisinopril and statin. Patient was given follow-up with cardiology and neurology and was recommended to get an MRI and echocardiogram (ECHO) with bubble study as an outpatient. At subsequent 2-week and 3-month follow-up visits, he remained in stable condition.
| Discussion | ▴Top |
ICA agenesis is an incredibly rare finding in patients with an incidence of approximately 0.01% [11]. Given the rarity of the condition and adaptive collateral circulation, most instances are identified incidentally. The case presented gives an overview of a possible presentation and subsequent management. The carotid arteries are vital vasculature that supplies the head and neck region of the body. The carotid arteries bifurcate into the ICA and ECA and both go on to supply the brain and neck/face respectively. Post-bifurcation, the ICA continues in the carotid sheath and enters the carotid canal through the temporal bone. The left and right ICAs eventually undergo anastomosis with the basilar artery and form the circle of Willis [2]. With the congenital absence of the ICA, the embryology surrounding the condition is also crucial in the formation of agenesis and collateral circulation. The ICA can be split into seven embryologic segments, cervical, ascending petrous, horizontal petrous, ascending cavernous, horizontal cavernous, clinoidal, and terminal, which can each develop agenesis [12]. Once the formation of these origins is present, the carotid canal usually forms by week 5 of gestation [13]. There are also various types of carotid artery agenesis with the patient presented above having the type A variant. Type A ICA agenesis is a unilateral absence of the ICA with collaterals to the anterior cerebral artery from the anterior communicating artery and the middle cerebral artery receiving circulation from the posterior communicating artery. In type B, the anterior and middle cerebral arteries are perfused by the anterior communicating artery. Type C involves bilateral agenesis and posterior vertebrobasilar circulation perfusing anterior areas. Type D contains unilateral agenesis of the cervical portion with the carotid siphon anastomosing to the contralateral cavernous ICA. Type E involved bilateral hypoplasia with minute anterior cerebral arteries and posterior circulation supplying the middle cerebral arteries. Lastly, type F has bilateral transcranial anastomoses of the distal ICA with the external carotids through branches of the internal maxillary artery [14]. In the patient case presented, there was also tortuosity of the basilar artery identified on imaging. This along with the hypertension on presentation and lack of a right ICA could have been a possible source of the patient’s symptoms. While there are possible mechanisms for compensation in congenital etiologies via the circle of Willis, having the lack of a carotid artery and comorbidities such as atherosclerosis may possibly lead to increased risk of developing neurological symptoms such as the ones presented in the above case.
In patients with ICA agenesis and the development of carotid artery stenosis due to atherosclerotic disease, there is an increased likelihood of TIA and a possible increased or earlier need for intervention if symptoms persist or worsen [4]. The literature suggests a link between reported cases of ICA agenesis and increased risk of cerebrovascular events [15]. While the cerebral arterial circle is an important source of collateral circulation known to reduce the risk of stroke in individuals, it is important to note that there is considerable anatomical variation between individuals and is not always complete [14]. ICA agenesis may impose additional constraints on individuals with varying levels of collateral circulation, leading to an increased risk of ischemic stroke in this patient population. For example, Szabo et al conducted a diffusion-weighted MRI study and found that different patterns of stroke were present depending on the degree of stenosis [15]. Additionally, in patients with ICA agenesis and overlying risk factors such as hypertension and hyperlipidemia, there is also a likelihood of stroke due to dissection of the ICA, especially in young patients who may not know of their ICA agenesis [5].
ICA agenesis can occur as an isolated vascular defect as seen in our case, but the literature points to a growing association with cerebral aneurysms, with an increased risk of 25-67% [16]. A study by Zink et al analyzed 165 cases with ICA aplasia or hypoplasia and found that nearly 28% of patients had associated cerebral aneurysms. Additionally, the fact there was a higher incidence of cerebral aneurysms in patients over 30 years old, suggests that these are not simply a concurrent structural anomaly, but rather an acquired etiology [17]. While the exact mechanism linking ICA agenesis with the increased risk of arterial aneurysms is not exactly known, one theory that has been proposed is that the lack of the ICA induces increased hemodynamic stress due to increased flow through collateral arteries which can, in turn, cause hemorrhage [16].
The vast implications of ICA agenesis for carotid endarterectomy, transsphenoidal surgery, and thromboembolic disease add to the emphasis on pre-testing and imaging to guide procedures [18]. The importance of successfully diagnosing a congenital ICA absence stems from the fact that there are risks of periprocedural stroke and late cerebrovascular events, including TIA and aneurysms [3]. The importance of recognizing and minimizing these risks in patients who have ICA agenesis further places importance on finding these vascular anomalies early on. Given the silent nature of ICA agenesis, screening and preventive measures may be a topic of further investigation and study.
The increased risk for cerebral aneurysm, paired with the potential sequelae of a life-threatening subarachnoid hemorrhage, brings up the question of whether there is a role for routine screening in individuals with known ICA agenesis. While there are important considerations when it comes to frequent imaging including radiation exposure, and patient costs, the benefits of possibly identifying an aneurysm before complications occur should not be overlooked. In a case report by Perla et al, a patient with ICA agenesis was followed for 7 years with annual imaging but no changes were discovered [19]. Given the nature of agenesis, risk factors such as hypertension, smoking, diabetes, hyperlipidemia, and cardiovascular disease should be closely followed and controlled. Age also plays an important role in carotid artery disease and as patients with known ICA disease progress, it is important to continue close follow-up [20]. However, without established guidelines, we recommend that brain aneurysm screening should be considered in the appropriate clinical context on an individualized basis.
Learning points
The primary learning points of this case report are to review the rare entity that is ICA agenesis, delineate underlying pathology and development of the vascular anomaly, including embryology and various forms, relate complications of the finding and possible further steps in monitoring and assessing patients who present with ICA agenesis. The types of ICA agenesis and hypoplasia play an important role in determining collateral circulation and provide places where aneurysms may develop. The risk of stroke and cerebral aneurysm also make it vital to recognize this anomaly early on in patient care and can sometimes explain symptoms such as in the case presented. Other complications such as TIA are also important for the patient and providers to know and recognize, in order to help minimize risk and improve patient outcomes. Lastly, the issue of surveillance and monitoring of these patients with ICA agenesis may be a topic for further research and discussion.
Conclusions
The data surrounding the clinical significance of ICA agenesis are scarce likely due to the incredibly rare nature of this condition. Typically, congenital absence of an ICA is often diagnosed incidentally during imaging of the cerebral vessels; it is important for clinicians to be aware of the findings and the potential implications of the anomaly. While ICA agenesis alone can be harmless, it is important for clinicians to be aware of the concurrent structural vascular abnormalities that are often seen including cerebral aneurysms. Furthermore, obtaining pertinent imaging and diagnostics for presentations of ICA atherosclerosis and TIA can help in possibly identifying and preventing future occurrences. This case report hopes to provide an overview of ICA agenesis along with the development, presentation, and complications of the condition to help improve overall patient outcomes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Verbal informed consent was obtained from the patient for their anonymized information to be published in this article.
Author Contributions
All authors contributed to writing the manuscript, literary search, and making edits. Ndausung Udongwo, Mohammad Hossain and Swapnil Patel were involved in study design and finding the case.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Zarrinkoob L, Ambarki K, Wahlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2015;35(4):648-654.
doi pubmed - Sethi D, Gofur EM, Munakomi S. Anatomy, head and neck, carotid arteries. In: StatPearls. Treasure Island (FL), 2022.
- DaCosta M, Tadi P, Surowiec SM. Carotid endarterectomy. In: StatPearls. Treasure Island (FL), 2022.
- Qaja E, Tadi P, Theetha Kariyanna P. Carotid artery stenosis. In: StatPearls. Treasure Island (FL), 2022.
- Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670.
doi pubmed - Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, Gray WA, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129(9):1048-1078.
doi pubmed - Sharma RK, Asiri AM, Yamada Y, Kawase T, Kato Y. Extracranial internal carotid artery aneurysm - challenges in the management: a case report and review literature. Asian J Neurosurg. 2019;14(3):970-974.
doi pubmed - Li S, Hooda K, Gupta N, Kumar Y. Internal carotid artery agenesis: A case report and review of literature. Neuroradiol J. 2017;30(2):186-191.
doi pubmed - Ryan FH, Kline LB, Gomez C. Congenital Horner's syndrome resulting from agenesis of the internal carotid artery. Ophthalmology. 2000;107(1):185-188.
doi - Goldman Lary B. Approach to cerebrovascular diseases. Goldman-Cecil Medicine, 26th ed. Elsevier; Philadelphia, PA. 2020; p. 2386-2396.
- Pasaoglu L, Vural M, Ziraman I, Uyaniotak SA. Left internal carotid artery agenesis associated with basilar and left vertebral artery aneurysm. J Clin Imaging Sci. 2011;1:60.
doi pubmed - Lasjaunias P, Santoyo-Vazquez A. Segmental agenesis of the internal carotid artery: angiographic aspects with embryological discussion. Anat Clin. 1984;6(2):133-141.
doi pubmed - Kathuria S, Gregg L, Chen J, Gandhi D. Normal cerebral arterial development and variations. Semin Ultrasound CT MR. 2011;32(3):242-251.
doi pubmed - Lamsal S, Burkins B, Matteo M, Matteo J, Harmon TS. A rare case of congenital internal carotid artery agenesis and contralateral internal carotid artery aneurysm. Cureus. 2022;14(3):e23619.
doi pubmed - Szabo K, Kern R, Gass A, Hirsch J, Hennerici M. Acute stroke patterns in patients with internal carotid artery disease: a diffusion-weighted magnetic resonance imaging study. Stroke. 2001;32(6):1323-1329.
doi pubmed - Takamiya S, Yoshimoto T, Maruichi K. Cerebral Aneurysms with Internal Carotid Artery Agenesis: A Unique Case Similar to Moyamoya Disease and Literature Review. Neurol Med Chir (Tokyo). 2021;61(5):321-333.
doi pubmed - Zink WE, Komotar RJ, Meyers PM. Internal carotid aplasia/hypoplasia and intracranial saccular aneurysms: series of three new cases and systematic review of the literature. J Neuroimaging. 2007;17(2):141-147.
doi pubmed - Given CA, 2nd, Huang-Hellinger F, Baker MD, Chepuri NB, Morris PP. Congenital absence of the internal carotid artery: case reports and review of the collateral circulation. AJNR Am J Neuroradiol. 2001;22(10):1953-1959.
- Perla FM, Carbotta G, Di Nardo D, D'Avanzo M, Colaiacomo MC, Di Biasi C, Falvo L, et al. Agenesis of the internal carotid artery: a family pathology? G Chir. 2017;38(1):46-49.
doi pubmed - Woo SY, Joh JH, Han SA, Park HC. Prevalence and risk factors for atherosclerotic carotid stenosis and plaque: A population-based screening study. Medicine (Baltimore). 2017;96(4):e5999.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


