| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 2, February 2023, pages 71-75
Durvalumab for Extensive-Stage of Small-Cell Lung Cancer With Lambert-Eaton Myasthenic Syndrome
Hirotomo Machiyamaa, Seigo Minamia, b
aDepartment of Respiratory Medicine, Osaka Police Hospital, Osaka, Japan
bCorresponding Author: Seigo Minami, Department of Respiratory Medicine, Osaka Police Hospital, Tennoji-ku, Osaka-City, Osaka 543-0035, Japan
Manuscript submitted December 24, 2022, accepted February 9, 2023, published online February 25, 2023
Short title: Durvalumab for Lambert-Eaton Syndrome
doi: https://doi.org/10.14740/jmc4043
| Abstract | ▴Top |
Durvalumab is an immune checkpoint inhibitor (ICI) of anti-programmed cell death protein 1 ligand antibody. ICI-combined chemotherapy has recently become a standard regimen for extensive-stage of small-cell lung cancer (ES-SCLC). SCLC is well known to be the most likely tumor associated with Lambert-Eaton myasthenic syndrome (LEMS), a rare autoimmune disease of a neuromuscular junction disorder. Although LEMS has been reported to be induced by ICI as immune-mediated adverse events, it remains unknown whether ICI can deteriorate preexisting paraneoplastic syndrome (PNS) of LEMS. Our rare case was successfully treated by durvalumab plus chemotherapy without exacerbation of preexisting PNS of LEMS. We report a 62-year-old female with ES-SCLC and preexisting PNS of LEMS. She started carboplatin-etoposide in combination with durvalumab. This immunotherapy achieved nearly complete response. However, multiple brain metastases were found after two courses of maintenance durvalumab. Her symptoms and physical examinations of LEMS improved despite of no significant change in compound muscle action potential amplitude in the nerve conduction study. The titer of anti-P/Q-type voltage-gated calcium channel (VGCC) antibody decreased from 1,419.2 to 263.5 pmol/L during the immunotherapy. In conclusion, ICI in combination with platinum doublet chemotherapy is still challenging but may be a treatment option for ES-SCLC patients complicated with PNS of LEMS.
Keywords: Lambert-Eaton myasthenic syndrome; Extensive-stage of small-cell lung cancer; Durvalumab; Immune checkpoint inhibitor; Immune-mediated adverse event; Paraneoplastic syndrome; Combination immunotherapy; Anti-P/Q-type voltage gated channel antibodies; Anti-programmed cell death protein 1 ligand antibody
| Introduction | ▴Top |
Lambert-Eaton myasthenic syndrome (LEMS) is a rare autoimmune disease of a neuromuscular junction disorder with typical clinical manifestations of proximal muscle weakness, decreased tendon reflexes and autonomic dysfunction [1]. More than half of the LEMS cases occur as a paraneoplastic syndrome (PNS), most commonly with small-cell lung cancer (SCLC) [2]. The anti-P/Q-type voltage-gated calcium channel (VGCC) antibody is detected in almost all cancer patients with LEMS, and in 91% of non-malignant patients with LEMS [3]. Thus, this antibody is a diagnostic biomarker for LEMS. When PNS of LEMS is found together with malignancy, cancer-directed treatment should be taken on the highest priority. Symptomatic treatments for LEMS include 3,4-diaminopyridine, which acts directly on the neuromuscular junction and is globally the leading treatment option. However, this drug remains not to be approved by Japanese medical insurance. Instead, pyridostigmine, an acetylcholine esterase inhibitor, is actually used for LEMS in Japan.
Durvalumab and atezolizumab are both immune checkpoint inhibitors (ICIs) of anti-programmed cell death protein 1 ligand (PD-L1) antibody. For untreated extensive stage (ES) of SCLC, these ICIs presented a revolutionary strategy of cancer immunotherapy by CASPIAN and IMpower133 phase 3 trials. The addition and maintenance of durvalumab and atezolizumab on and after platinum plus etoposide significantly improved overall survival in patients with ES-SCLC [4, 5]. We should be careful of various immune-mediated adverse events (imAEs), including rare but severe neurological disorders. We previously reported the first case of imAE of LEMS caused by nivolumab, anti-PD-1 antibody, in a 73-year-old and heavily pretreated Japanese woman with c-stage IV of pulmonary squamous cell carcinoma [6]. Our previous case suggested LEMS as a result of imAE due to ICI. On the other hand, it remains unknown whether ICI may deteriorate underlying and preexisting PNS of LEMS.
We report a patient with ES-SCLC and pre-existing LEMS, who was treated with durvalumab-combined with platinum-doublet chemotherapy regimen.
| Case Report | ▴Top |
Investigations
A 62-year-old Chinese woman living in Japan suffered from weakness of lower extremities after accidental employment injury of comminuted fracture of the right ankle in September 2020, which lead to edema and pain of her lower extremities, gait disturbance and repeated falls. She also developed ptosis, diplopia, and tinnitus in February 2021. She was hospitalized in the Department of Neurology of another hospital in April 2021 because of sudden onset of dizziness, tinnitus, abdominal pain and cold sweat at home. In the admission, her serum anti-acetylcholine receptor and anti-muscle-specific kinase antibodies were both negative. She was referred to the Department of Respiratory Medicine of our hospital in June 2021 because of mediastinal tumors and left hilar lymphadenopathy on chest computed tomography (CT) (Fig. 1a). She was a current smoker with a 24 pack-year smoking history. She had a past history of pulmonary tuberculosis in 30s with unknown details and had no other significant medical history but hypertension at that time.
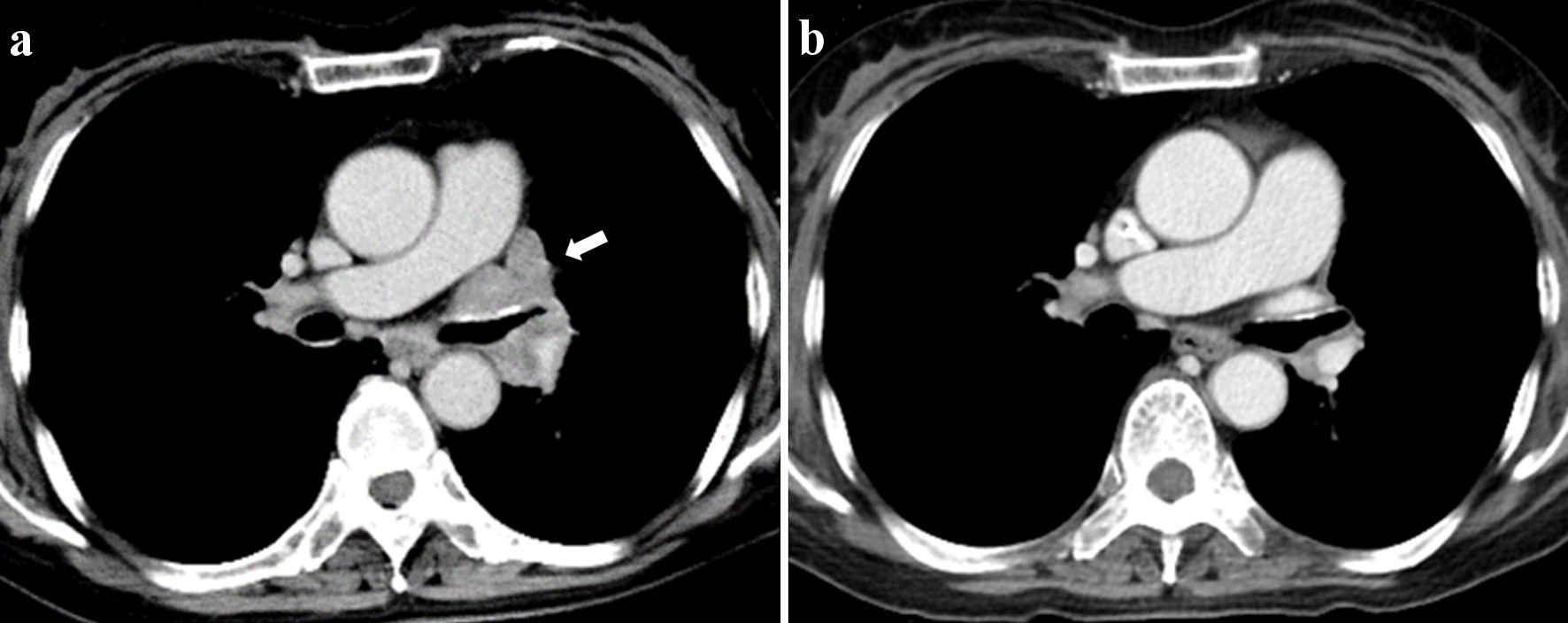 Click for large image | Figure 1. Chest CT before the initiation of treatment (a) and after two courses of treatment (b). White arrow indicates pretreatment left hilar lymphadenopathy. CT: computed tomography. |
Diagnosis
She underwent endobronchial ultrasound-guided trans-bronchial needle aspiration (EBUS-TBNA) from the right hilar lymph node and was pathologically diagnosed as SCLC (Fig. 2). Her serum pro-gastrin releasing peptide (Pro-GRP) value showed elevated up to 496 pg/mL. Contrast-enhanced CT detected a pancreatic metastasis. Thus, her clinical stage was determined as c-stage IVA (cTxN3M1b).
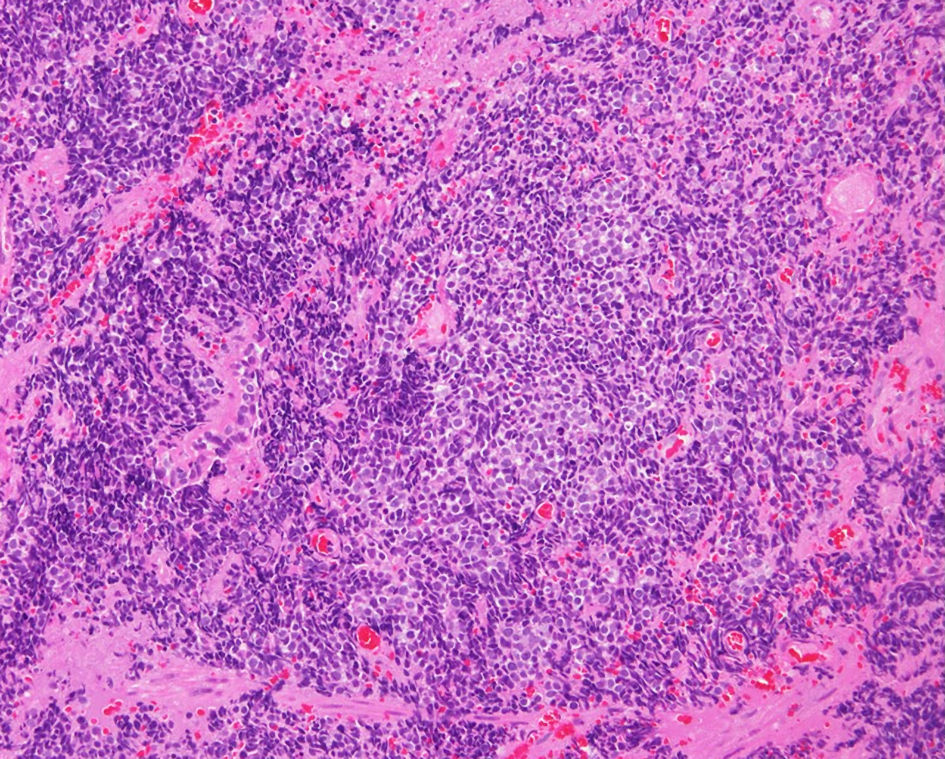 Click for large image | Figure 2. Histopathological finding of endobronchial ultrasound-guided trans-bronchial needle aspiration biopsy from the right hilar lymph node (hematoxylin and eosin stain). |
On her neurologic examination, she presented bilateral proximal limb weakness (manual muscle test (MMT) 3 - 4), decreased deep tendon reflexes, disability in the daily activities with modified Rankin scale (mRS) of 4, and a positive edrophonium responsiveness with disappearance of her ptosis. Nerve conduction studies of the left median, ulnar and tibial nerves detected reduced compound muscle action potential (CMAP) amplitude at rest, and then showed five-fold (from 0.7 mV at resting to 3.5 mV after exercise), 2.4 (1.2 to 2.9 mV) and 7.1 (0.7 to 5.0 mV) increases after 20 s exercise, respectively (Fig. 3a). Repetitive nerve stimulation of the left ulnar nerve showed a waning phenomenon with 3 Hz repetitive stimulation (Fig. 3b) and waxing phenomenon with 30 Hz repetitive stimulations (Fig. 3c). Electroencephalogram did not find epileptiform discharges. Based on these neurological findings, she was diagnosed as PNS of LEMS. Thereafter, the titer of anti-P/Q-type VGCC antibodies was found to be elevated up to 1,419.2 pmol/L in her serum.
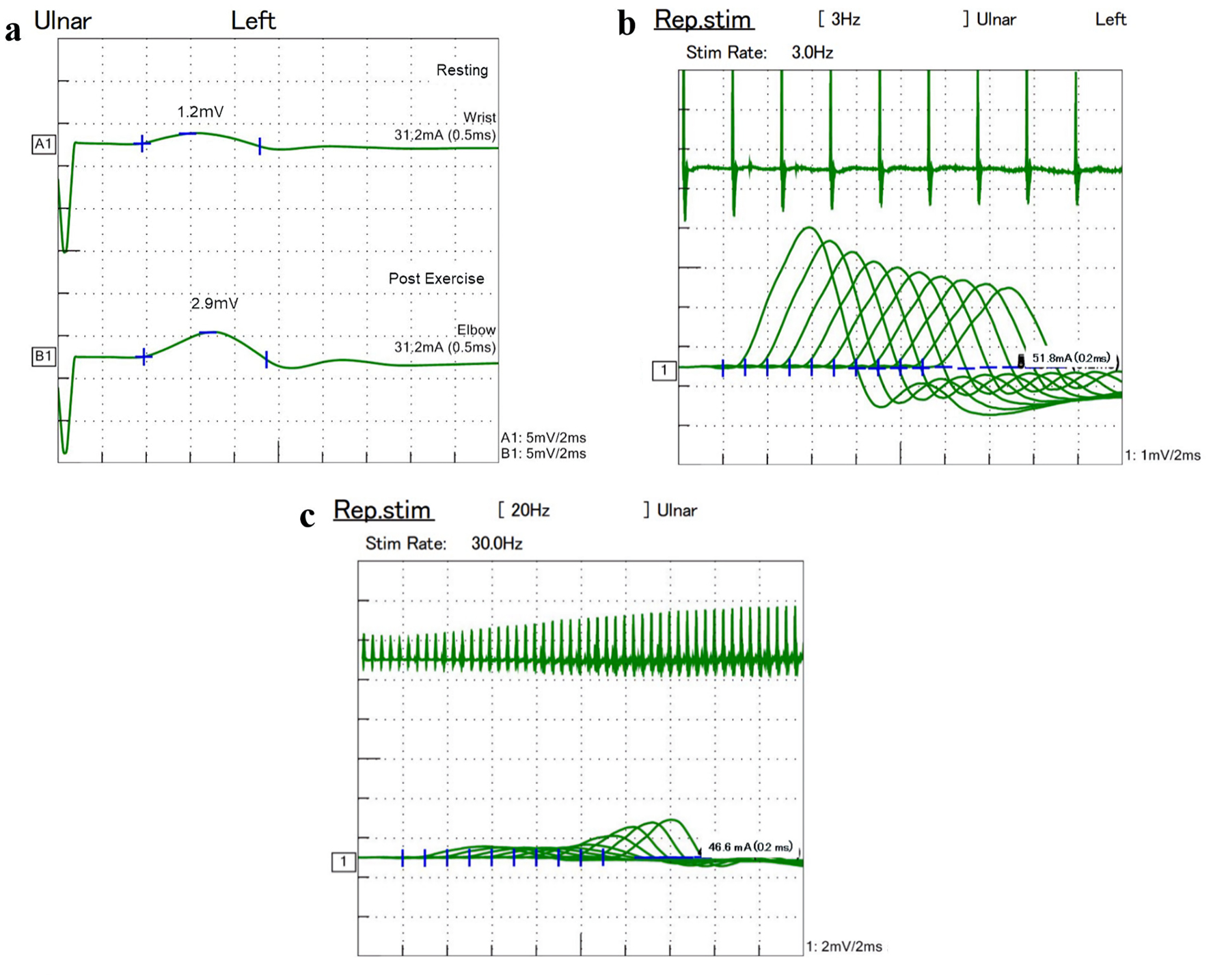 Click for large image | Figure 3. Nerve conduction study showed 2.4-fold increase of CMAP amplitude after exercise (a). Repetitive nerve stimulation of the left ulnar nerve presented waning phenomenon with 3 Hz repetitive stimulation (b) and waxing phenomenon with 30 Hz repetitive stimulation (c). CMAP: compound muscle action potential. |
Treatment
We administered pyridostigmine for the purpose of improving the symptoms of LEMS. On the other hand, we proposed two regimen options for ES-SCLC; conventional platinum-based chemotherapy alone or anti-PD-L1 inhibitor combined with chemotherapy. We obtained her informed consent after thorough explanation of the possible risk of exacerbating her LEMS and the survival benefits of immunotherapy. She started triweekly combination immunotherapy of carboplatin (area under the curve 5 mg/mL/min, day 1) and etoposide (80 mg/m2, day 1 - 3) with durvalumab (1,500 mg/body, day 1). Four courses of this regimen achieved nearly complete response (Fig. 1b). Pro-GRP decreased from 496 pg/mL before the treatment down to 49.8 pg/mL after four courses of the induction immunotherapy.
Follow-up and outcomes
After two courses of durvalumab maintenance, we found multiple brain metastases, despite of normal range of Pro-GRP (56.2 pg/mL). She received whole brain irradiation and then amrubicin as the second-line regimen. She was still alive and continuing chemotherapy 1.5 years after the diagnosis without exacerbation of LEMS.
Six months after the initiation of the first-line combined immunochemotherapy, when we confirmed disease progression, her CMAP amplitudes did not change significantly in the nerve conduction study. Nerve conduction studies of the left median, ulnar and tibial nerves showed 4.1- (from 1.5 mV at resting to 6.1 mV after exercise), 3.5- (0.85 to 3.0 mV) and 1.6-fold (2.6 to 4.2 mV) increases after exercise, respectively. However, her decreased deep tendon reflexes recovered. Her proximal limb weakness and disability in the daily activities also improved to MMT 5 or 5-, and mRS of 1, respectively. The titer of anti-P/Q-type VGCC antibodies decreased to 263.5 pmol/L.
| Discussion | ▴Top |
This is the first case of durvalumab for ES-SCLC with PNS of LEMS. In our case, combined immunochemotherapy did not exacerbate the preexisting LEMS. To our knowledge, there were only four malignant cases who had preexisting LEMS but had been treated by ICIs [7-10]. These rare cases suggest that ICI is a possible treatment option for cancer patients with LEMS.
The most important finding of our case was that four induction courses of durvalumab plus platinum-based chemotherapy and two additional courses of durvalumab maintenance did not exacerbate the preexisting LEMS. Reviewing the previous four cases, ICIs remain controversial for preexisting PNS of LEMS (Table 1). LEMS improved in two cases but deteriorated in the other two cases. Among five cases, one Swedish and one Japanese case presented different clinical courses of LEMS. In the Swedish case, LEMS was already improved at the introduction of avelumab for Merkel cell carcinoma by the previous treatment of local irradiation and cytotoxic chemotherapy. These treatments reduced the titer of anti-P/Q-type VGCC antibodies from 65.2 pmol/L at the diagnosis to undetectable level at the initiation of ICI. In this case, LEMS did not deteriorate by 1.5-year administration of avelumab, which was discontinued because of imAEs of hypothyroidism and drug-induced pneumonitis [9]. The Japanese case by Takigawa et al [7] presented a 73-year-old woman who maintained complete remission from localized stage of SCLC associated with LEMS 22 years earlier. Her LEMS recurred by only four courses of pembrolizumab with carboplatin and nab-paclitaxel for c-stage IIIB of pulmonary squamous cell carcinoma. However, her neurological symptoms were improved by discontinuation of pembrolizumab and intravenous immunoglobulin therapy. Then, she could continue chemotherapy without pembrolizumab. On the other hand, ours and the other two cases introduced ICIs soon after the confirmed diagnosis of malignancy and worsening LEMS [8, 10]. Among these three cases, LEMS improved by combination immunotherapy in two Japanese cases, but deteriorated by ICI monotherapy in the other German case. Our case was very similar in clinical course, treatment regimen and outcomes to the other Japanese case by Sakaguchi et al [8]. The difference between these two Japanese cases was ICI drug, i.e., durvalumab vs. atezolizumab. In contrast, LEMS exacerbated in the German case, which dared to continue avelumab concurrently with intravenous immunoglobulins and kept complete remission of Merkel cell carcinoma [10]. In our opinion, ICI is not absolute contraindication, but a possible treatment option for cancer patients with preexisting stable LEMS, because flare-up of LEMS was manageable in the previous cases.
 Click to view | Table 1. Summary of Previous Cases of ICIs for Cancers With LEMS |
The second important finding was that only our case followed the titer of anti-P/Q-type VGCC antibody and confirmed the remarkable decrease of this serum biomarker before and after the immunotherapy. As a result, we observed significant improvement of symptoms and physical examinations, despite of no remarkable change of CMAP amplitudes and detectable level of the remaining anti-P/Q-type VGCC antibody. We should be still careful of flare-up of LEMS, because we did not confirm complete disappearance of antibody biomarker. However, our monitoring results may encourage oncologists to dare to use ICI for PNS of LEMS. Thus, ICI is not strongly recommended and remains challenging for malignancy with PNS of LEMS, but can be considered as a treatment option.
In conclusion, we successfully treated an ES-SCLC patient complicated with preexisting PNS of LEMS by durvalumab plus chemotherapy without a flare-up of LEMS. We also confirmed the remarkable decrease of the serum titer of anti-P/Q-type VGCC antibody. ICI in combination with platinum doublet chemotherapy is still challenging but may be a treatment option for ES-SCLC patients complicated with PNS of LEMS.
Learning points
This case highlights no harmful influence of durvalumab on the preexisting stable PNS of LEMS. The main take-away points from this case report is that ICI is a possible treatment option for cancer patients with preexisting LEMS. According to the previous cases, flare-up of LEMS can be managed by immunoglobulin.
Acknowledgments
We thank Dr. Yoko Ohoka and Dr. Kazuo Hashikawa (Department of Neurology, Osaka Police Hospital) for their contributions to the diagnosis and consultation of this patient.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have no conflict of interest to declare.
Informed Consent
Not applicable because the manuscript has been sufficiently deidentified to protect the patient.
Author Contributions
All authors were involved in diagnosis, treatment, and management of this patients. H. Machiyama drafted the report. All authors read and critically reviewed the manuscript, and then approved the final submitted version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CMAP: compound muscle action potential; CT: computed tomography; ES-SCLC: extensive-stage of small-cell lung cancer; ICI: immune checkpoint inhibitor; imAE: immune-mediated adverse event; LEMS: Lambert-Eaton myasthenic syndrome; MMT: manual muscle test; mRS: modified Rankin scale; PD-L1: programmed cell death protein 1 ligand; PNS: paraneoplastic syndrome; Pro-GRP: pro-gastrin releasing peptide; VGCC: voltage-gated calcium channel
| References | ▴Top |
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10(12):1098-1107.
doi pubmed - Schoser B, Eymard B, Datt J, Mantegazza R. Lambert-Eaton myasthenic syndrome (LEMS): a rare autoimmune presynaptic disorder often associated with cancer. J Neurol. 2017;264(9):1854-1863.
doi pubmed - Lennon VA, Kryzer TJ, Griesmann GE, O'Suilleabhain PE, Windebank AJ, Woppmann A, Miljanich GP, et al. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med. 1995;332(22):1467-1474.
doi pubmed - Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939.
doi pubmed - Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229.
doi pubmed - Nakatani Y, Tanaka N, Enami T, Minami S, Okazaki T, Komuta K. Lambert-eaton myasthenic syndrome caused by nivolumab in a patient with squamous cell lung cancer. Case Rep Neurol. 2018;10(3):346-352.
doi pubmed - Takigawa Y, Watanabe H, Omote Y, Kurihara S, Inoue T, Fujiwara M, Mitsumune S, et al. Lambert-eaton myasthenic syndrome recurrence induced by pembrolizumab in a patient with non-small-cell lung cancer. Intern Med. 2022.
doi pubmed - Sakaguchi T, Kokubo Y, Furuhashi K, Nakamura Y, Suzuki Y, Ito K, Fujiwara K, et al. An extensive-stage small-cell lung cancer case with preexisting lambert-eaton myasthenic syndrome successfully treated with an immune checkpoint inhibitor. Clin Lung Cancer. 2022;23(3):e273-e275.
doi pubmed - Green C, Isaksson Mettavainio M, Kjellman C, Ramqvist T, Dalianis T, Israelsson P, Lindquist D. Combined treatment with radiotherapy, chemotherapy and avelumab results in regression of metastatic Merkel cell carcinoma and improvement of associated Lambert-Eaton myasthenic syndrome: A case report. Oncol Lett. 2022;24(5):393.
doi pubmed - Dohrn MF, Schone U, Kuppers C, Christen D, Schulz JB, Gess B, Tauber S. Immunoglobulins to mitigate paraneoplastic Lambert Eaton Myasthenic Syndrome under checkpoint inhibition in Merkel cell carcinoma. Neurol Res Pract. 2020;2:52.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


