| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 2, February 2023, pages 54-58
Severe Hemolytic Disease of the Newborn due to Anti-Dia
Douglas Blackall
Department of Pathology and Laboratory Services, Providence Health and Services, Portland, OR 97213, USA
Manuscript submitted January 4, 2023, accepted February 7, 2023, published online February 25, 2023
Short title: Anti-Dia Hemolytic Disease of the Newborn
doi: https://doi.org/10.14740/jmc4047
| Abstract | ▴Top |
Dia is a low-frequency member of the Diego blood group system, which is comprised of 23 antigens. The Diego blood group antigens are found on the erythroid membrane glycoprotein band 3, the red cell anion exchanger (AE1). The behavior of anti-Dia in pregnancy can only be surmised by rare, published case reports. This is a case report of severe hemolytic disease of the newborn due to a high-titer maternal anti-Dia immune response. The neonate’s mother was monitored throughout pregnancy with Dia antibody titers. In the third trimester, her antibody titer abruptly rose to 32. Her fetus was emergently delivered and was found to be jaundiced at birth with a hemoglobin/hematocrit of 5 g/dL/15.9% and a neonatal bilirubin of 14.6 mg/dL. With simple transfusion, intensive phototherapy, and two doses of intravenous immunoglobulin, the neonate’s condition normalized quickly. He was discharged from the hospital after 8 days in excellent condition. Anti-Dia is uncommonly encountered in both transfusion services and obstetric practices. Although very rare, anti-Dia can be associated with cases of severe hemolytic disease in newborns.
Keywords: Anti-Dia; Dia antibody; Diego blood group system; Hemolysis; Hemolytic disease of the newborn
| Introduction | ▴Top |
The Diego blood group system is comprised of 23 blood group antigens, three of which are high prevalence and 20 of which are low prevalence [1]. The Dia antigen was first identified in 1955, when the immune response to this antigen (in Mrs. Diego) was implicated in a fatal case of hemolytic disease of the fetus and newborn (HDFN) in Venezuela [2]. Dia is a low-frequency antigen in the Diego system, with a prevalence of 0.01% in most populations [3]. However, there is a wide geographic variation in prevalence, with some groups having a higher incidence of the antigen including North and South American Indigenous populations (2-54%), Japanese (12%), Chinese (5%), and Polish (about 0.5%) [3]. As another example, in a South Texas blood donor population, 2.6% of donors were found to be Dia positive [4]. Although the Diego antigens, and the immune responses to these antigens, are of greatest medical interest to transfusion medicine specialists, the variation in Dia antigen prevalence among differing populations has had significant implications in anthropology. The Dia antigen was the first, and possibly most convincing, genetic marker linking Amerindians to Siberia [5].
The generally low prevalence Dia antigen has an antithetical “partner” in the high prevalence Dib antigen, which is present in nearly 100% of all populations studied [5]. A single base substitution (rs2285644C>T, Chr17:42328631) defines two alleles, DI*B and DI*A, which are responsible for the Dib and the Dia antigens, respectively [5]. Antibodies to both Dia and Dib are rare in most populations studied as those who are Dia negative are only rarely exposed to Dia antigens through either transfusion or pregnancy. Similarly, those lacking the Dib antigen are rare, so it is very uncommon to identify Dib antibodies. As such, very little is known about the immune responses to these blood group antigens and their clinical significance with respect to hemolysis (hemolytic transfusion reactions and cases of HDFN). These are largely limited to rare case reports with respect to anti-Dia in particular [6-9]. As such, it is important to understand these antigens, and their accompanying immune responses, so that strategies can be implemented to prevent hemolytic consequences. What can be said with certainty, through published case reports, is that Dia and Dib antibodies can be clinically significant with respect to hemolysis. This case report clearly indicates that anti-Dia can cause severe HDFN and should be taken very seriously in the pregnant patient with this antibody.
| Case Report | ▴Top |
Investigations
A 35-year-old pregnant woman presented to her obstetrician for a first prenatal visit at about 8 weeks gestation. She had no significant past medical or surgical history and had never been transfused. She had an extensive obstetric history, however, including an ectopic pregnancy, two spontaneous abortions, and a term pregnancy with a normal spontaneous vaginal delivery. She always had negative antibody screens during her pregnancies. In addition, her husband was the father of all five of her pregnancies.
Routine prenatal testing was unremarkable except for her immunohematology evaluation. The patient was blood group O-positive and had a moderately reactive antibody screen with solid phase testing (Immucor; Peachtree Corners, GA). Antibody identification testing revealed anti-Kidda (Jka) with a 1-2+ strength of reactivity (0-4+ scale). However, there was a single Jka-negative cell that was 4+ reactive. This cell was Dia positive, so anti-Dia was suspected. This was confirmed by an immunohematology reference lab. At that time, the Jka antibody was too weak to titer, but the Dia antibody had a titer of 8. Of note, antibody titration was performed using a test tube-based agglutination methodology that included an anti-IgG secondary antibody. Given the patient’s complex findings, she was referred to a maternal-fetal medicine specialist who recommended monthly antibody titers. In the meantime, the father of the fetus underwent testing for Kidd and Diego antigens. He typed Jk(a+b-), which predicted a 100% chance that the fetus would be Jka-positive. Diego genotyping revealed the father to be DI*01/02, with a predicted Diego phenotype of Di(a+b+). As such, the fetus would have a 50% chance of being Dia-positive. Of special note, the mother of the fetus was white and not of Hispanic or Latino origin. The father of the fetus was Hispanic, his family of origin being from southern Mexico.
The mother of the fetus had a normal pregnancy in all respects. Her fetus grew as expected and exhibited normal movement throughout pregnancy. The patient’s Jka antibody remained too weak to titer throughout the course of the pregnancy. The Dia antibody titer remained low, in the 4 - 8 range throughout pregnancy until the final determination. At about 37 weeks gestation, the Dia antibody was unexpectedly stronger, with a titer of 32. Because of this, labor was induced. The mother had a normal spontaneous vaginal delivery of a male neonate with Apgar scores of 8 and 9 at 1 and 5 min, respectively.
Diagnosis
The neonate weighed 3,140 g (about 6 pounds, 15 ounces) at birth, was vigorous, did not require advanced resuscitation, and had normal cord blood gas parameters. Approximately 3 h after delivery, however, he was noted to be jaundiced and had laboratory evidence of severe hemolysis (neonatal reference ranges are noted parenthetically; all values were obtained at 5 h of life): neonatal bilirubin of 14.6 mg/dL (0 - 6), hemoglobin of 5 g/dL (10.1 - 22.0), hematocrit of 15.9% (33.0-66.0%), mean corpuscular volume (MCV) of 134.7 fL (98.0 - 118), nucleated red blood cells of 134/100 white blood cells (WBCs) (0/100 WBCs), reticulocytes of 19.4% (2.5-6.5%), and an absolute reticulocyte count of 229.0 × 109/L (22.8 - 159.3). The neonate had normal platelet and WBC counts, though neutrophil progenitor cells (a left shift) and numerous erythroid progenitor cells were identified on peripheral blood smear review (Fig. 1). Based on these findings, umbilical artery and vein catheters were placed in preparation for an exchange transfusion for presumed severe HDFN.
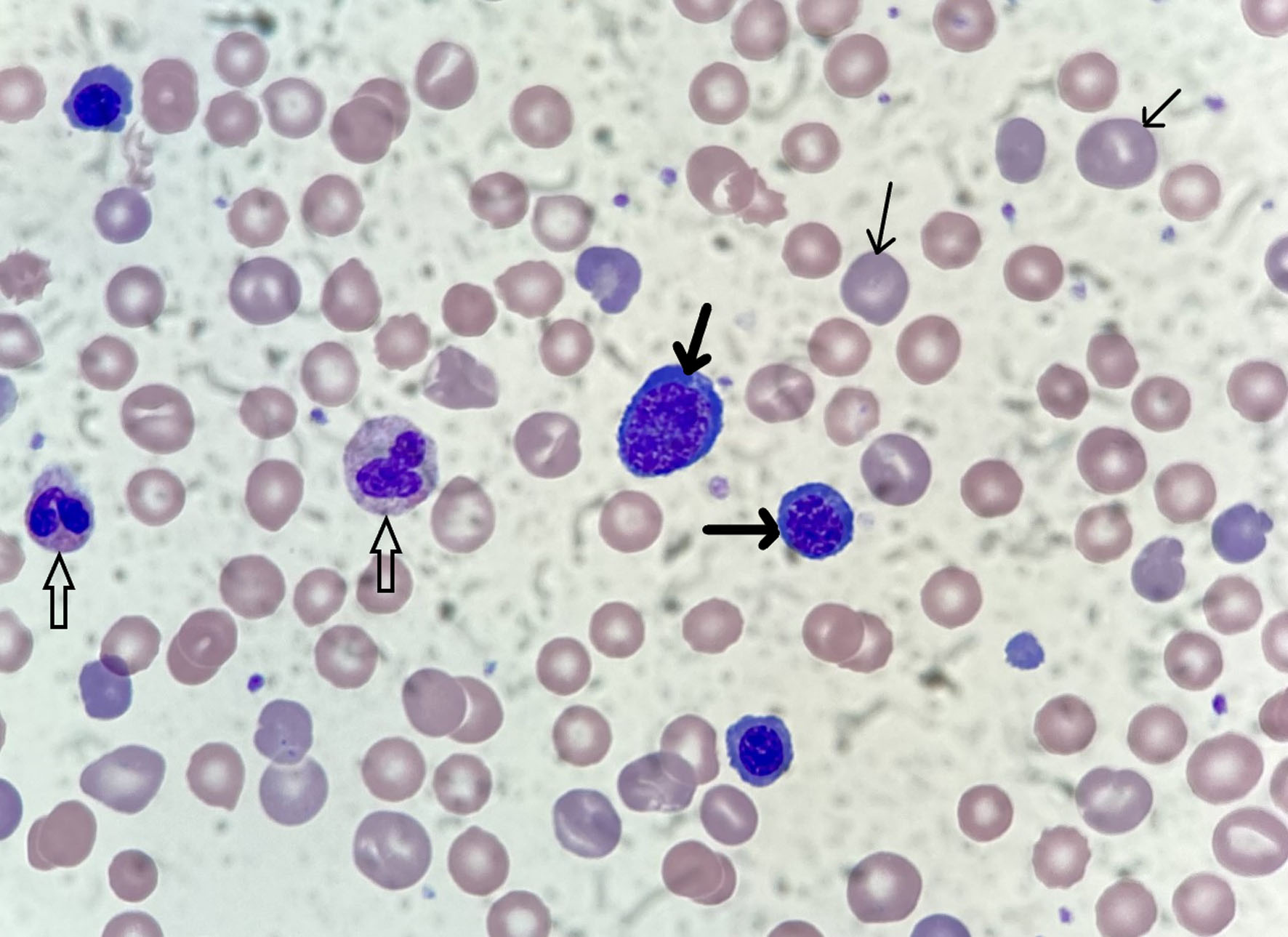 Click for large image | Figure 1. Photomicrograph (× 1,000 magnification) of the neonate’s peripheral blood smear on day 1 of life. Many polychromatophilic cells are seen (thin arrows) along with nucleated red blood cells at various maturational stages (thick arrows). Two neutrophils are also present (open arrows). |
Treatment
Intensive phototherapy was initiated (four overhead blue LED lights emitting in the 450 - 475 nm spectrum plus a bilirubin blanket - a portable phototherapy device consisting of an LED pad placed in direct contact with the neonate’s skin), and a dose of intravenous immunoglobulin (IVIG) was administered (1 g/kg; 3.15 g dose). The neonate also received an emergency transfusion (20 mL/kg) of group O red cells that were Jka negative and compatible with mother’s plasma (i.e., presumed to be Dia-negative since Dia typing sera is not readily available, but mother had high-titer anti-Dia). Blood bank evaluation revealed that the neonate was A-positive. Testing of neonate’s plasma (solid phase methodology) revealed anti-Dia, while anti-Jka was not detected. The direct antiglobulin test (DAT) demonstrated 4+ reactivity with anti-IgG as assessed by column agglutination technology (Ortho Clinical Diagnostics; Raritan, NJ). An eluate revealed that anti-Dia was bound to the neonate’s red blood cells, but neither anti-Jka nor anti-A were detected. As such, a diagnosis of severe HDFN was made secondary to maternal anti-Dia.
The neonate received a second red cell transfusion (20 mL/kg) and a second dose of IVIG. His neonatal bilirubin peaked at 17.3 mg/dL (7 h of life) but fell to 15.8 mg/dL 2 h later. As such, an exchange transfusion was not undertaken. With simple transfusion, the neonate’s hemoglobin and hematocrit rose initially to 14.9 g/dL and 41.4%, respectively, and increased to 18.2 g/dL and 52.0% without additional transfusion therapy. He required continued, intensive phototherapy, but this was gradually withdrawn as his neonatal bilirubin values fell. At 12 h of life, the neonatal bilirubin was 13.0 mg/dL, and it was 11.3 mg/dL at 24 h of life. At 7 days of life, phototherapy was discontinued as the neonatal bilirubin had normalized to 10.2 mg/dL.
Follow-up and outcomes
The neonate was discharged on day 8 of life with a neonatal bilirubin of 10.5 mg/dL, a hemoglobin of 14.3 g/dL, and a hematocrit of 42.4%. The neonate had gained weight throughout his hospitalization and was discharged to his home in excellent condition. One week after hospital discharge, the neonate’s neonatal bilirubin was 11.6 mg/dL. As this laboratory value appeared to be stable, no further testing was performed. However, for approximately the next 3 months, at 1- to 2-week intervals, a blood sample was obtained to assess the infant’s hemoglobin/hematocrit and reticulocyte count. For the first 2 months, the reticulocyte count remained slightly elevated in the 2.8-3.6% range, but the infant experienced significant anemia with a hemoglobin of about 9.0 g/dL and a hematocrit of about 25% (nadir of 8.4 g/dL and 23.2%). Eventually, in the third month after delivery, the infant’s reticulocyte count normalized (1.9%) and his hemoglobin/hematocrit stabilized (10.4 g/dL and 28.6%). He did not require transfusion after hospital discharge. At his 12-month check-up, the infant was doing well, had successfully reached all developmental milestones, and had average cognitive, language, and motor scores for his age.
| Discussion | ▴Top |
Because it is such a low incidence antigen in most populations, anti-Dia is rarely encountered on the transfusion service. Even when it is present in pregnancy, it may not be readily identified, until time of birth, as the red blood cells used in antibody screening (red cell samples from 2 to 3 individual donors of known blood group antigen phenotype) typically do not carry the Dia antigen. Anti-Dia may, however, be detected in the context of an antibody identification panel (red cells from 10 to 20 donors), which is undertaken when the antibody screen is positive, as most current panels will contain one Dia-positive red cell. In this case, maternal anti-Dia was identified because: 1) the antibody screen was reactive due to anti-Jka (a common blood group antibody of known potential consequence) and 2) there was a Jka-negative, Dia-positive red cell on the antibody identification panel that was strongly reactive. In short, without a positive antibody screen attributable to anti-Jka, which proved to be clinically inconsequential, anti-Dia might not have been identified so early in pregnancy, which proved to be clinically significant. Unfortunately, it is highly likely that most cases of anti-Dia HDFN will only be identified at time of delivery, when a baby is born jaundiced, anemic, and has a positive DAT, since antibody screening cells rarely carry low-prevalence blood group antigens such as Dia.
Although Dia antibodies are not well-characterized immunologically, they are typically immune stimulated (i.e., by transfusion or pregnancy-related red blood cell exposure) and are of the IgG1 and IgG3 subclasses [10]. As such, they readily cross the placenta, have an extended half-life of 21 days, have strong avidity for Fcγ receptors on macrophages, but are inefficient activators of the complement system [11]. These features predict that high-titer Dia antibodies could result in HDFN, especially as the Diego antigens are expressed well on fetal and neonatal red blood cells [12]. Inefficient complement binding also helps to explain why Diego antibodies are infrequently implicated in hemolytic transfusion reactions.
As this case demonstrates, anti-Dia can be a cause of severe HDFN. There are several other case reports that corroborate this finding, but Dia antibodies remain a rare cause of HDFN. Interestingly, there are significantly more case reports of HDFN due to anti-Dib, which can help to shed light on Dia antibodies and their clinical behavior [13-15]. An older case report (2006) of anti-Dib HDFN included an extensive literature review that identified 27 additional reports of this entity [16]. The authors classified the cases into three groups according to their severity, as defined by the treatment of the neonate that was required. Ten of the neonates required no therapy (mild or no evidence of HDFN), six of the neonates required phototherapy alone (moderate HDFN), and 11 neonates required exchange transfusion and/or high-dose IVIG therapy plus phototherapy (severe HDFN). The authors further noted a significant correlation between maternal anti-Dib titer and the severity of HDFN. Namely, the mothers of all neonates with severe disease had an anti-Dib titer of 64 or greater. Since the Dia and Dib antigens are very similar biochemically (they are defined by a single amino acid polymorphism, p.Leu854 for Dia and p.Pro854 for Dib) and immunologically (Dib antibodies, like Dia antibodies, are typically IgG1 or IgG3) [10], it seems reasonable to extrapolate what we know of anti-Dib HDFN to anti-Dia HDFN. In short, HDFN due to anti-Dia can range from clinically inconsequential to highly clinically significant and the titer of the maternal antibody correlates directly with the risk of HDFN severity. The present case bears this out as the maternal antibody titer abruptly rose to 32, and the neonate had evidence of severe HDFN shortly after delivery.
Because the neonate was severely anemic and hyperbilirubinemic within 5 h of delivery, an emergency exchange transfusion was planned using red blood cells that had been prepared for the patient’s mother (group O red cells lacking the Dia and Jka blood group antigens). Since these were immediately available, they were used for simple transfusion to the neonate (two occasions). At the same time, intensive phototherapy and IVIG administration were provided. The rationale for these interventions was to 1) acutely raise the neonate’s critically low red blood cell mass (transfusion), 2) stabilize and decrease the neonate’s elevated bilirubin value (phototherapy), and 3) reduce ongoing hemolysis of neonatal red blood cells (IVIG). In tandem, these interventions worked extremely well as the neonate’s hemoglobin increased with transfusion (5 to 14.9 g/dL) and his bilirubin peaked at 7 h of life (17.3 mg/dL) and trended down thereafter. The success of these interventions avoided the need for exchange transfusion.
The role of IVIG in this treatment strategy is particularly interesting and provocative. The putative therapeutic effect of IVIG is that it binds to and blocks the Fcγ receptors on macrophages which reduces the destruction of antibody-coated red blood cells, in this case neonatal red cells coated with maternal anti-Dia [17]. A number of clinical trials suggest that IVIG is efficacious in the setting of HDFN [18, as an example], but its use remains somewhat controversial as these studies had a moderate to high risk of bias, and the majority were undertaken prior to the institution of high-intensity phototherapy [17]. IVIG therapy itself can cause hemolysis and has been associated with necrotizing enterocolitis, but it may represent a potent therapeutic tool, especially if it can obviate the need for exchange transfusion, which is associated with significant complications (e.g., catheter-related infections, hypocalcemia, thrombosis, hemorrhage, cardiorespiratory instability, necrotizing enterocolitis, and mortality, up to 5%) [17].
A final interesting aspect of this case relates to the ongoing anemia that the infant experienced in the first 3 months of life. After hospital discharge, his reticulocyte count remained elevated and he was anemic, with a nadir hemoglobin of 8.4 g/dL. Although the precise mechanism for these findings is unclear, and may in fact be multifactorial, ongoing, lower-level hemolysis seems likely, especially given the high titer of the maternal IgG anti-Dia and its relatively long half-life in the circulation (21 days). In addition, some blood group antibodies are known to inhibit erythropoiesis and may be responsible for a later phase of anemia in HDFN that is not classically hemolytic [19]. To the author’s knowledge, it is unknown whether Diego antibodies have been shown to inhibit erythropoiesis. Regardless, it is important to closely monitor neonates who have experienced significant HDFN, as was done in this case, to assure that their hemoglobin values do not fall to critically low levels with the necessity for additional transfusion therapy.
Conclusion
Although very rare, maternal Dia antibodies are associated with cases of severe HDFN. As such, women with these antibodies should be followed closely throughout their pregnancies. Their neonates should also be monitored for hemolysis and treated as indicated.
Learning points
The Diego blood group system is comprised of 23 discreet antigens, the immune response to which may result in hemolytic transfusion reactions and HDFN. Anti-Dia is a rare blood group antibody, but it has been implicated in cases of HDFN of varying severity. The combination of simple transfusion, intensive phototherapy, and prompt IVIG administration may obviate the need for exchange transfusion. Infants who are treated successfully for severe HDFN should be monitored carefully after hospital discharge to address the possibility of late-phase anemia.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
A waived consent form was approved according to our institution’s medical research requirements.
Author Contributions
DB formulated and wrote the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
DAT: direct antiglobulin test; Dia/Dib: Diegoa/Diegob; HDFN: hemolytic disease of the fetus and newborn; IVIG: intravenous immunoglobulin; Jka: Kidda
| References | ▴Top |
- Gassner C, Castilho L, Chen Q, Clausen FB, Denomme GA, Flegel WA, Gleadall N, et al. International Society of Blood Transfusion Working Party on Red Cell Immunogenetics and Blood Group Terminology Report of Basel and three virtual business meetings: Update on blood group systems. Vox Sang. 2022;117(11):1332-1344.
doi pubmed - Layrisse M, Arends T, Dominguez SR. Nuevo grupo sanguineo encontrado en descendientes de Indios. Acta Medica Venez. 1955;3:132-138.
- Meunier D, Peng S, Clarke G. Diego system: anti-Dia. [Internet] Ottawa: Canadian Blood Services; 2019 Sept 25 [cited 2023 01 03]. Available from: https://profedu.blood.ca/en/transfusion/best-practices/serological-best-practices.
- Thompson C. Diego(a) antigen frequency and anti-Diego(a) frequency in a South Texas community. Clin Lab Sci. 2006;19(4):203-205.
- Begat C, Bailly P, Chiaroni J, Mazieres S. Revisiting the Diego blood group system in Amerindians: evidence for gene-culture comigration. PLoS One. 2015;10(7):e0132211.
doi pubmed - Ting JY, Ma ES, Wong KY. A case of severe haemolytic disease of the newborn due to anti-Di(a) antibody. Hong Kong Med J. 2004;10(5):347-349.
- Mun SH, Lee SH, No MY. A case of acute hemolytic transfusion reaction due to anti-Di(a) antibody -A case report. Korean J Anesthesiol. 2012;63(4):353-356.
doi pubmed - Jethava A, Olivares E, Shariatmadar S. A case of hemolytic disease of the newborn due to Di (a) antibody. Case Rep Pediatr. 2015;2015:897803.
doi pubmed - Fu Y, Liu Y, Yang Z, An Y, Su J, Hu S, Luo L. Neonatal hemolytic disease due to anti-Diego(a) antibody: a case report. J Med Case Rep. 2022;16(1):274.
doi pubmed - Melland C, Nance S. Other blood group systems and antigens. In: Cohn CS, Delaney M, Johnson ST, et al, eds. AABB Technical Manual, 20th ed. Bethesda, MD: AABB. 2020; p. 355-388.
- Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520.
doi pubmed - Reid ME, Lomas-Francis C. Diego blood group system. In: Reid ME, Lomas-Francis C, eds. The Blood Group Antigen FactsBook. San Diego, CA: Academic Press. 1997; p. 232-244.
- Lenkiewicz B, Zupanska B. The first example of anti-Diego(b) found in a Polish woman with the Di(a+b-) phenotype and haemolytic disease of the newborn not requiring treatment. Transfus Med. 2003;13(3):161-163.
doi pubmed - Oh EJ, Jekarl DW, Jang HS, Park HI, Park YJ, Choi HA, Chun CS, et al. Severe hemolytic disease of the newborn due to anti-Di b treated with phototherapy and intravenous immunoglobulin. Ann Clin Lab Sci. 2008;38(1):80-82.
- Avila Rueda JA, Acosta J, Tonietto C, Rabinovich O. Identification of antibodies against Diego B in the context of a hemolytic disease of the newborn. J Appl Hematol. 2021;12:109-111.
doi - Mochizuki K, Ohto H, Hirai S, Ujiie N, Amanuma F, Kikuta A, Miura S, et al. Hemolytic disease of the newborn due to anti-Di: a case study and review of the literature. Transfusion. 2006;46(3):454-460.
doi pubmed - Lieberman L, Lopriore E, Baker JM, Bercovitz RS, Christensen RD, Crighton G, Delaney M, et al. International guidelines regarding the role of IVIG in the management of Rh- and ABO-mediated haemolytic disease of the newborn. Br J Haematol. 2022;198(1):183-195.
doi pubmed - Vlachodimitropoulou E, Lo TK, Bambao C, Denomme G, Seaward GR, Windrim R, Tessier F, et al. Intravenous immunoglobulin in the management of severe early onset red blood cell alloimmunization. Br J Haematol. 2023;200:100-106.
doi pubmed - Luban NL. Hemolytic disease of the newborn: progenitor cells and late effects. N Engl J Med. 1998;338(12):830-831.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


