| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 3, March 2023, pages 88-94
Extracranial Microvascular Complications of Moyamoya Disease Leading to Left Ventricular Systolic Dysfunction
Sohiub N. Assafa, c, Abdallah N. Assafa, Muaz N. Assafa, John Taylorb, Justin M. Adama, Justin Harrellb, Jeffery Johnsonb
aDepartment of Medicine, The University of Tennessee Graduate School of Medicine, Knoxville, TN 37920, USA
bDepartment of Cardiovascular Disease, The University of Tennessee Graduate School of Medicine, Knoxville, TN 37920, USA
cCorresponding Author: Sohiub N. Assaf, Department of Medicine, The University of Tennessee Graduate School of Medicine, Knoxville, TN 37920, USA
Manuscript submitted February 6, 2023, accepted March 13, 2023, published online March 31, 2023
Short title: Complications of MMD
doi: https://doi.org/10.14740/jmc4057
| Abstract | ▴Top |
Moyamoya disease (MMD) was first used as a descriptor for a steno-occlusive process that affects primarily the internal carotid arteries (ICA) in a bilateral fashion in 1969. Characterized by recurrent ischemic events in the developing brains of young patients, the process is one that often decimates the quality of life of affected individuals. The vascular changes in MMD have been demonstrated to occur in an extracranial manner, thus it is logical to assume that the same steno-occlusive mechanism could induce dysfunction and ischemia in other organ systems. Our case presents a patient with MMD with cardiac manifestations that we suspect may be related to extracranial manifestations of MMD.
Keywords: Moyamoya disease; Congestive heart failure; Heart failure with reduced ejection fraction; Non-ST elevated myocardial infarction; Systemic inflammatory response syndrome
| Introduction | ▴Top |
Moyamoya disease (MMD) was first used as a descriptor for a steno-occlusive process that affects primarily the internal carotid arteries (ICA) in a bilateral fashion in 1969 [1]. Characterized by recurrent ischemic events in the developing brains of young patients, the process is one that often decimates the quality of life of affected individuals [1]. Its pathophysiology has been further characterized as the formation of microvascular collaterals in response to smooth muscle hyperplasia and luminal thrombosis which reduces blood flow through the ICA’s and their proximal branches [2]. The cerebrovascular implications of MMD are widespread and include focal neurological symptoms in the frontal, parietal, and temporal lobes, migraine-like headaches, and choreiform movement disorder [3, 4]. The latter is an example of the disease’s tendency to induce pathology in a pattern of microvascular damage in the basal ganglia [5]. The vascular changes in MMD have been demonstrated to occur in an extracranial manner, thus it is logical to assume that the same steno-occlusive mechanism could induce dysfunction and ischemia in other organ systems [6-10].
The pathophysiological process of arterial stenosis and small vessel collateralization involves vessel wall thickening and angiogenesis. MMD has genetic association with a higher incidence among the Japanese population with familial occurrence as high as 15%. Evidence suggests that RNF213 gene on chromosome 17q25.3 is an important susceptibility factor for the development of MMD in east Asian populations [11]. MMD is associated with multiple conditions that may be causative or syndromic in association. These include diseases affecting arteries around the circle of Willis, hematological conditions (sickle cell disease, beta-thalassemia), vasculitis and autoimmune and multisystem diseases (systemic lupus erythematosus, polyarteritis nodosa), genetic and developmental disorders (Alagille syndrome, Down syndrome), other vasculopathies and extracranial cardiovascular diseases (coarctation of the aorta, fibromuscular dysplasia), and metabolic diseases and renal disorders (type I glycogenosis, polycystic kidney disease) [12, 13].
Our case highlights a young female with apparent coronary artery disease that was suspected to be related to the rare extracranial manifestations of MMD. This case presents the rare instance of possible cardiac manifestations rarely associated with the typical sequela MMD.
| Case Report | ▴Top |
Investigations
A 37-year-old woman with known MMD presented to our medical center with complaints of altered mental status, lethargy, and hypotension. The patient also endorsed mild dyspnea and nonproductive cough. The patient denied associated palpitations, syncope, or fever.
Initial vitals showed a blood pressure of 101/69 mm Hg, heart rate of 66 beats per minute, respiratory rate of 12 per minute, and SpO2 of 100% on 2 L nasal cannula. Physical examination showed bilateral lower extremity pitting edema, and flaccid paralysis of the bilateral upper and lower extremities. The patient was alert and orient to person but not to place or time. The remainder of the physical examination was unremarkable.
The patient’s past medical history included MMD which was initially diagnosed in 2016 after suffering intraparenchymal hemorrhage (IPH) which involved the basal ganglia. The patient underwent extensive evaluation to assess the underlying cause of the sudden cerebral insult. The diagnosis was confirmed after magnetic resonance angiography (MRA) and cerebral angiogram revealed stenosis of the right and left internal carotid arteries as well as the vertebral arteries and anterior cerebral arteries. The patient underwent a combination of balloon angioplasty and bilateral internal carotid artery stent placement. It was determined at the time of diagnosis that the patient had MMD with stage III involvement according to the Suzuki staging model [14]. The patient had no significant family history. Home medications included aspirin 81 mg daily, gabapentin 600 mg twice daily alongside duloxetine, tramadol, and omeprazole.
During the current admission, an initial transthoracic echocardiogram (TTE) was performed that confirmed a left ventricular systolic function that was severely reduced, and left ventricular ejection fraction (LVEF) was 23% by the biplane method of disks alongside apical akinesis. TTE imaging also revealed a moderate size apical left ventricular thrombus (Fig. 1). The left atrium was normal in size, and there was no evidence of mitral stenosis or mitral regurgitation. The aortic valve was normal in structure and function with no hemodynamically significant aortic stenosis or aortic regurgitation. The right ventricular function was moderately reduced with evidence of a hypokinetic apex and right ventricular free wall. There was trace tricuspid regurgitation with a right ventricular systolic pressure (RVSP) estimated at 19.0 mm Hg (Fig. 1, Supplementary Videos 1-3, www.journalmc.org). At that time, the differential diagnosis included ischemic cardiomyopathy. Computerized tomography angiography (CTA) of the chest with contrast showed no evidence of coronary calcification. Two prior echocardiographic evaluations, completed in the preceding 18 months, showed an LVEF of 55% with no evidence of regional wall abnormalities. The patient was admitted, and further imaging was obtained. The patient was subsequently started on intravenous (IV) heparin 25,000 units (18 units/kg/h).
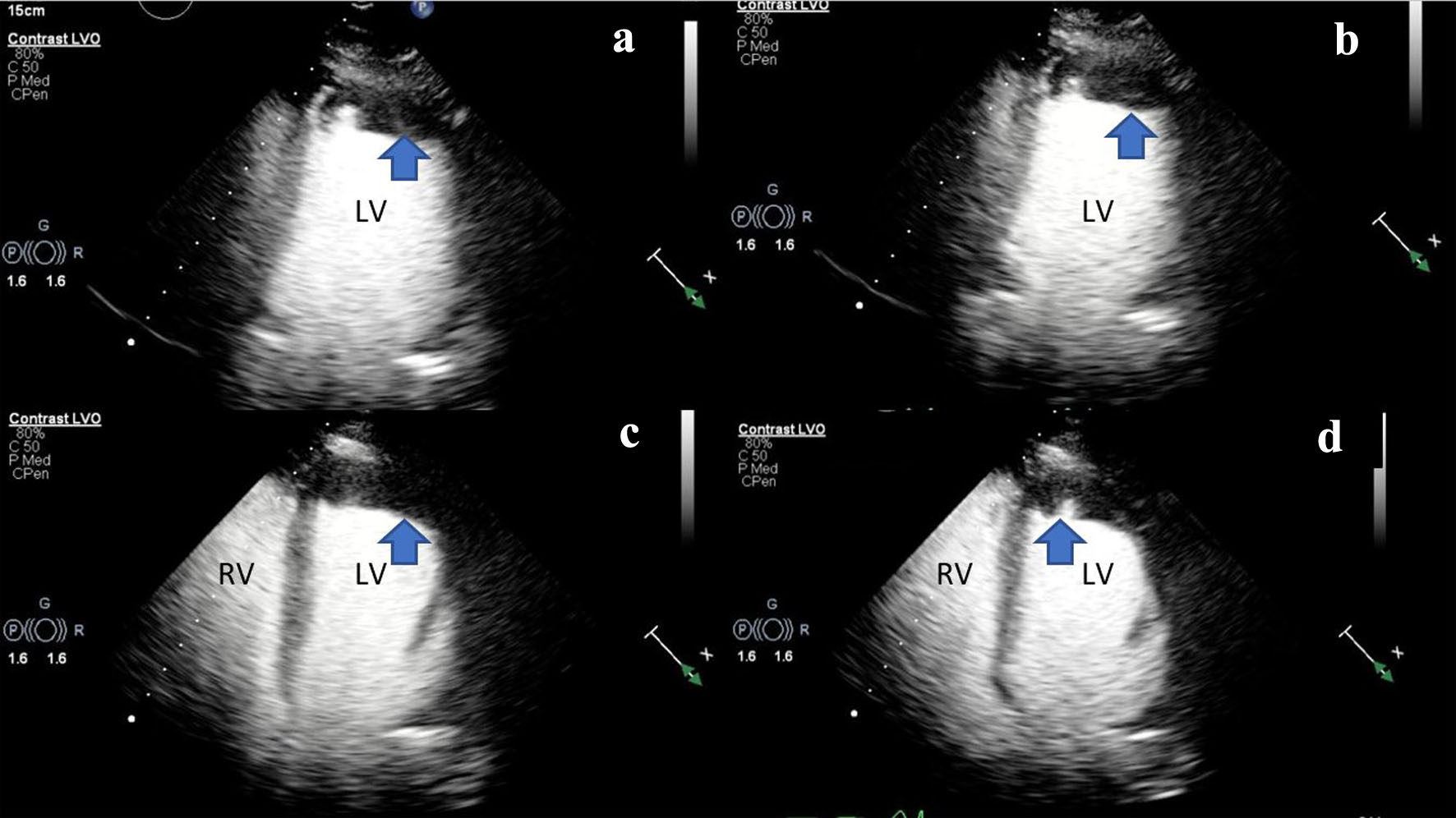 Click for large image | Figure 1. (a, b) Two-dimensional TTE (ultrasound enhancing agents) of an apical two-chamber view, at end-systole demonstrating severely reduced LV systolic function and LV apical hypokinesis with an associated LV apical thrombus (blue arrow). (c, d) Two-dimensional TTE still frame (ultrasound enhancing agents) of an apical four-chamber view, at mid-systole demonstrating biventricular dilation and an LV apical thrombus (blue arrow). Left ventricular internal diameter in diastole (LVIDd): 5.3 cm (3.7 - 5.5 cm), left ventricular internal diameter in systole (LVIDs): 4.7 cm (2.0 - 4.0 cm), right ventricular internal diameter in diastole (RVDd): 3.7 cm (1.9 - 3.5 cm). LV: left ventricle; RV: right ventricle; TTE: transthoracic echocardiogram. |
Diagnosis
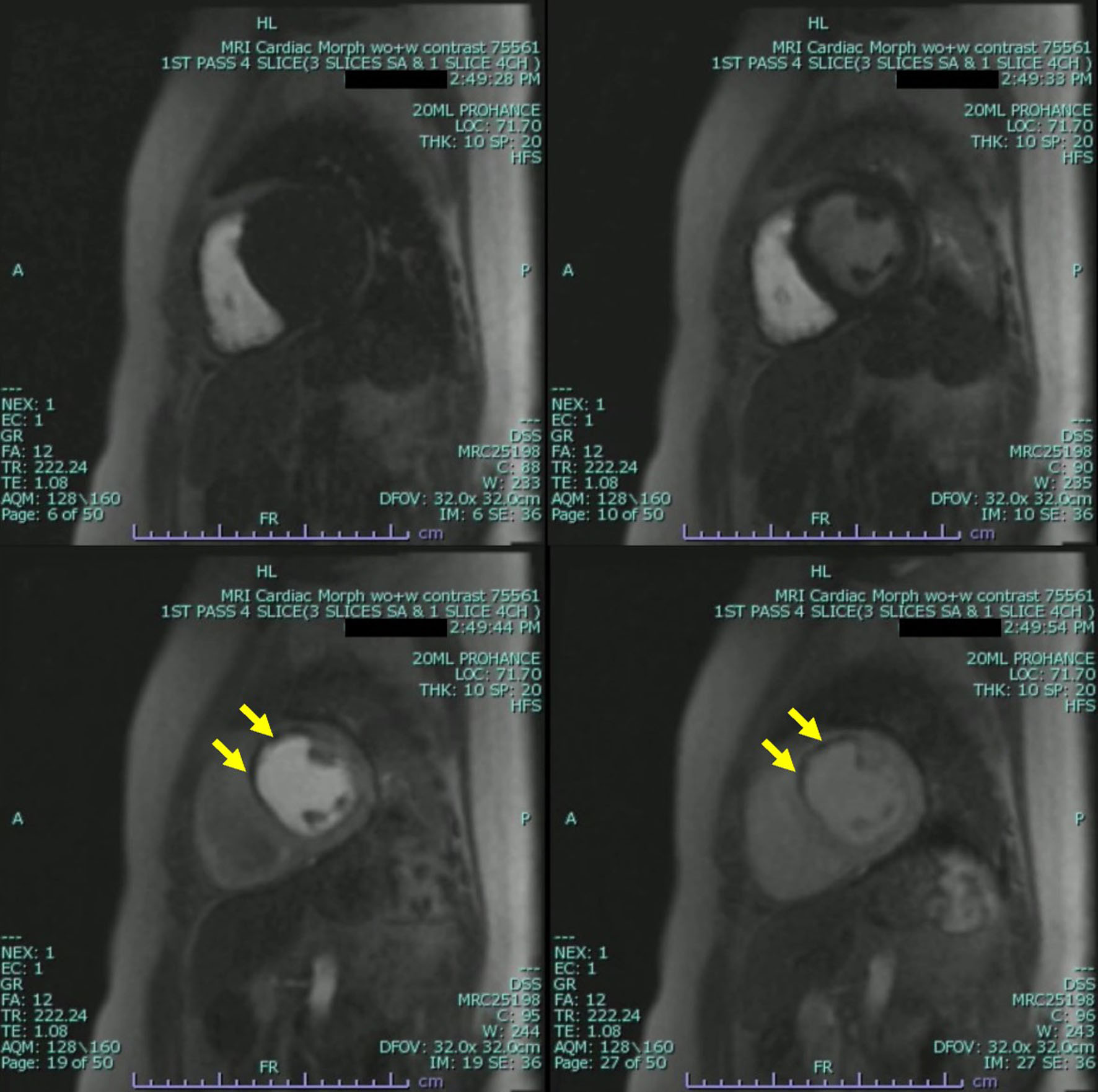
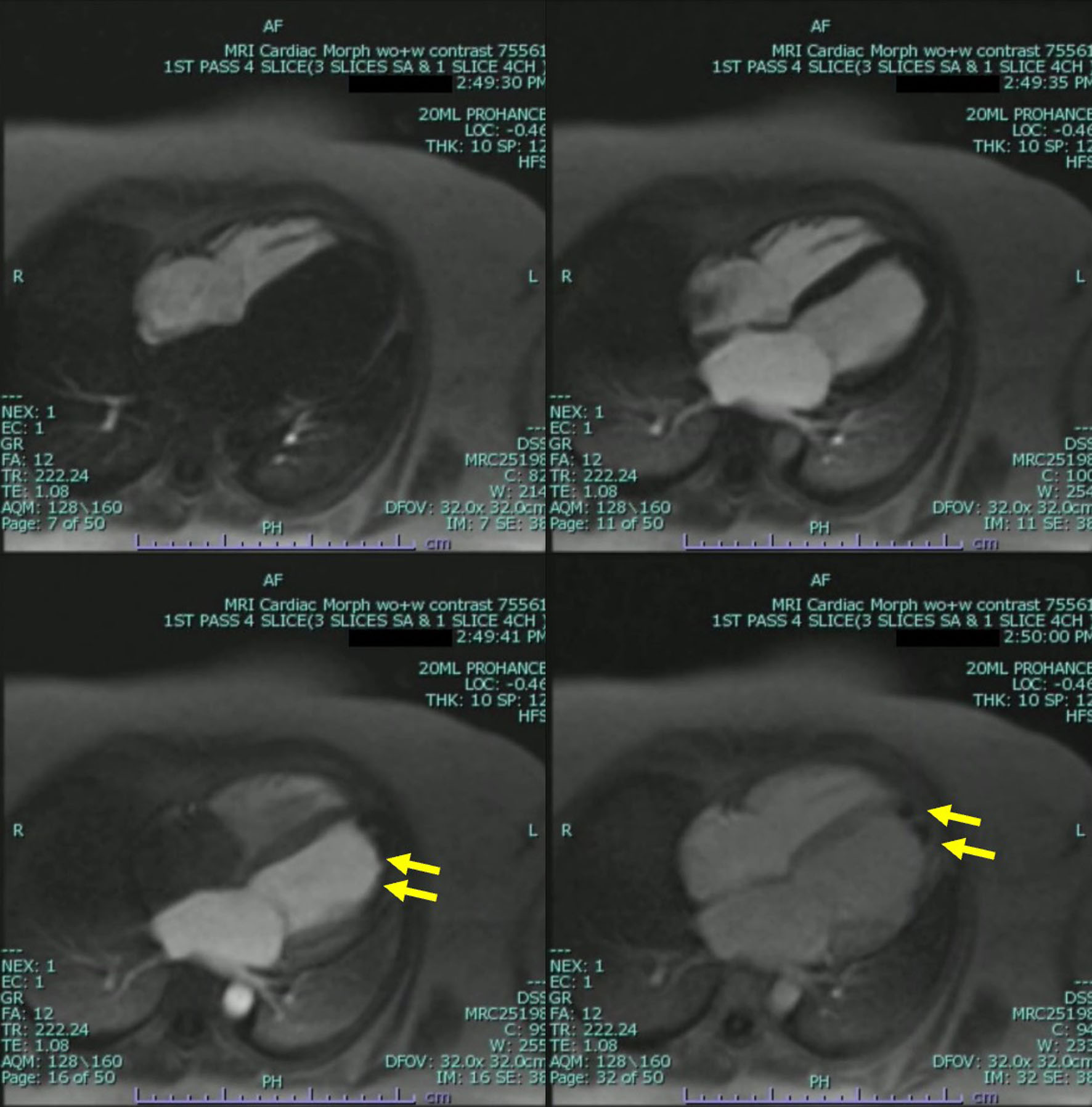
The patient underwent further evaluation with a cardiovascular magnetic resonance imaging (CMR) in order to better characterize the nature of the left ventricular mass and assess for any underlying ischemic disease contributing to the patient’s presentation. CMR remonstrated a large thrombus measuring 3.0 × 1.2 cm in the apex and apical anterior wall alongside a normal left ventricular size with severe systolic dysfunction, LVEF 22%. There was severe hypokinesis of the anterior wall and akinesis of the apex and apical lateral wall. Imaging also showed near transmural late gadolinium enhancement of the apex, transmural late gadolinium enhancement in the apical and mid lateral wall as well as greater than 75% subendocardial late gadolinium enhancement in the apical and mid anterior wall (Fig. 2). This was consistent with an infarction pattern with no significant viability in these territories. Right ventricular imaging showed a normal right ventricular size with severe systolic dysfunction, right ventricular ejection fraction (RVEF) 13% with mild bi-atrial enlargement (Figs. 3, 4, Supplementary Videos 4-7, www.journalmc.org).
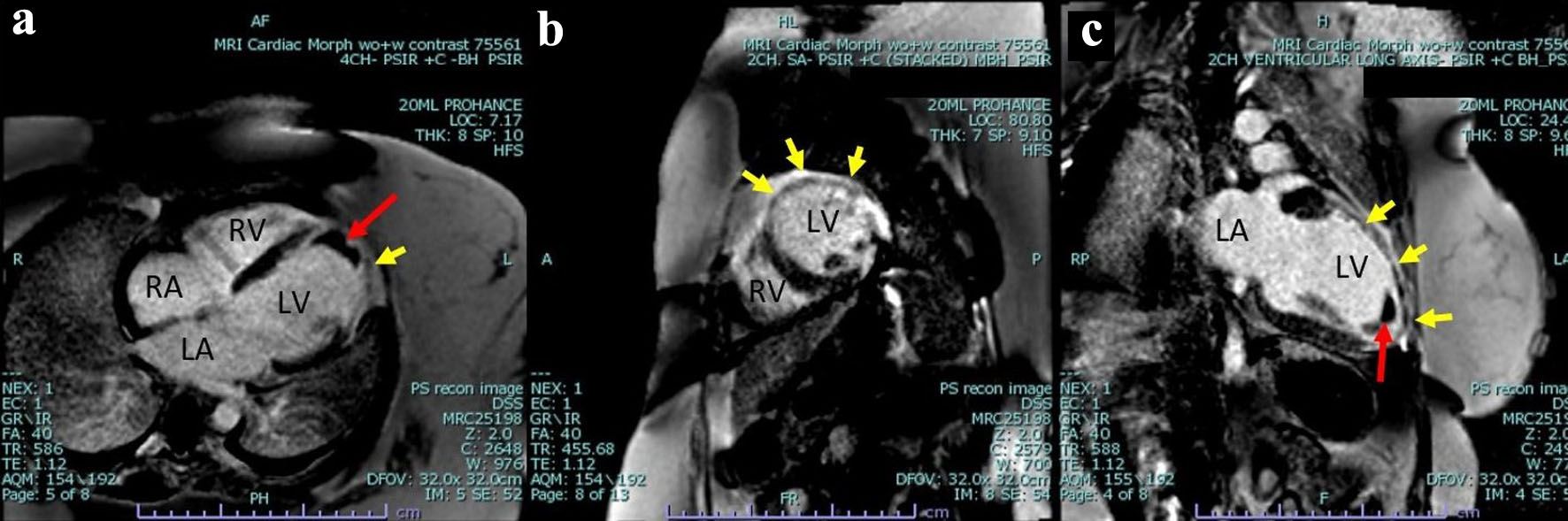 Click for large image | Figure 2. Cardiac magnetic resonance imaging phase-sensitive inversion recovery (CMRI PSIR) contrast-enhanced tissue always displays a higher signal (lighter) than normal myocardium (darker). (a) Four-chamber view, large thrombus measuring 3.0 × 1.2 cm in the apex and apical anterior wall (red arrow) as well as greater than 75% subendocardial late gadolinium enhancement in the mid anterior wall (yellow arrow). (b) Two-chamber short axis view demonstrating near transmural late gadolinium enhancement of the mid lateral wall transmural as well as greater than 75% subendocardial late gadolinium enhancement in the mid anterior wall (yellow arrow). (c) Two-chamber ventricular long axis view demonstrating a large thrombus measuring 3.0 × 1.2 cm in the apex and apical anterior wall (red arrow), as well as near transmural late gadolinium enhancement of the mid lateral wall transmural as well as greater than 75% subendocardial late gadolinium enhancement in the mid anterior wall and apex (yellow arrow). LV: left ventricle; RV: right ventricle; RA: right atrium; LA: left atrium. |
 Click for large image | Figure 3. Cardiac magnetic resonance imaging (CMRI) two-chamber short axis first-pass perfusion imaging sequences still frame with injection of gadolinium demonstrating absence of uptake on first-pass perfusion of the mid anterior wall consistent with infarct pattern (yellow arrow). |
 Click for large image | Figure 4. Cardiac magnetic resonance imaging (CMRI) two-chamber short axis first-pass perfusion imaging sequences still frames with injection of gadolinium demonstrating absence of uptake on first-pass perfusion of the apical and mid lateral wall consistent with infarct pattern. Apical mass is also demonstrated with absence of uptake on first-pass perfusion indicating a mass without native blood supply consistent with a thrombus (yellow arrow). |
Treatment
A multidisciplinary meeting was arranged in order to evaluate the patients’ appropriateness for coronary angiography, and it was determined that it would not be in the best interest of the patient to pursue further invasive testing. This plan was formed based on a shared decision making with the family and the multidisciplinary team. The patient lacked capacity to give informed consent due to prior debilitating CVA. The patient was scheduled to undergo cardiac CT in the outpatient setting. The patient was continued on warfarin, aspirin, and high-intensity statin therapy. The patient was continued on carvedilol, sacubitril/valsartan, spironolactone, and furosemide for medical management optimization.
Follow-up and outcomes
Patient was subsequently lost to follow-up. No further outpatient evaluations or testing was conducted.
| Discussion | ▴Top |
Our case presents a young female with evidence of ischemic cardiac disease that was suspected to be attributed to extracranial manifestations of MMD. Initial workup showed evidence of a severely depressed ejection fraction alongside evidence of a left ventricular aneurysm and thrombus. Further investigation showed evidence of regional wall motion abnormalities that was suspected and later confirmed to be due to underlying ischemic disease that we suspected to be related to extracranial steno-occlusive manifestation of MMD which is typically associated with cerebrovascular manifestations primarily. This presentation is atypical in a young female and highlights the importance of evaluating the possibility of unordinary manifestations of a disease usually associated with cerebrovascular events.
The pathogenesis of MMD is related to impaired inflammatory response or defects in cellular repair mechanisms. These changes have been associated with increased angiogenesis-related factors, high levels of fibroblast growth factor, which may stimulate arterial growth, have been found in the vascular intima, media, and smooth muscle as well as cerebrospinal fluid among patients with MMD [15]. Vessel abnormalities characterized by narrowed or blocked blood flow often occur in the major blood vessels in the circle of Willis and in the smaller collateral vessels in people with MMD. These abnormalities may be caused by an accumulation of fibrous and cellular material in the inner lining of the vessels, as well as by changes in the internal elastic structure and thinning of the middle layer of the vessels. A defining feature of MMD is the development of a network of enlarged and dilated small arteries as a means of compensating for the impaired blood flow. In addition to affecting the intracranial vessels, stenosis caused by thickening of the inner lining of the vessels may also occur in the extracranial and systemic arteries, including the cervical carotid, and renal arteries.
Although involvement of the coronary blood supply is not typically associated with the sequelae of MMD, the well-described extracranial involvement does raise the question of whether coronary involvement of MMD may be the inciting contributor in our patients’ presentation especially in the absence of risk factors for coronary artery disease. This hypothesis is driven by the presence of apparent infarcted cardiac tissue on cardiac MRI alongside the development of severely reduced cardiac function and an aneurysmal ventricular wall. There have been reports of patient’s coronary involvement in MMD with cases ranging from stenosis of the coronary arteries to provocation-induced vasospasm of the arteries (Table 1 [14, 16-26]). Two patients presented with reduced ejection fractions such as ours and one patient presented with symptoms of heart failure but was expired on arrival [17, 21, 27]. It is difficult to assess the exact etiology of our patient coronary involvement as cardiac angiography was not conducted.
 Click to view | Table 1. Cases of Moyamoya Disease Associated With Coronary Artery Disease, Adapted From Komiyama et al [14] |
Conclusion
MMD is a complex disease associated with the pathophysiological process of arterial stenosis and small vessel collateralization that is classically associated with cerebrovascular events related to the underlying pathological arterial architecture. Although rarely described in the literature, MMD can have associated extracranial manifestations specifically those with involvement in the coronary artery architecture. This coronary involvement may contribute to a host of disease complications and as described in our case ischemic cardiac disease. We highlight this case and provide a comprehensive literature review of previous cases with similar manifestations to help clarify possible atypical manifestations of this complex disease.
Learning points
This case highlights the possibility of coronary artery manifestations in a patient with known MMD. Although not readily described in the literature, MMD does have a broad extracranial involvement that we believe may extend to the coronary arteries.
| Supplementary Material | ▴Top |
Video 1. Two-dimensional TTE of a parasternal long axis view demonstrating severely reduced LV systolic function and mild to moderate reduction in RV systolic function. TTE: transthoracic echocardiogram; LV: left ventricular; RV: right ventricular.
Video 2. Two-dimensional TTE of a parasternal short axis view demonstrating severely reduced LV systolic function and mild to moderate reduction in RV systolic function.
Video 3. Two-dimensional TTE (ultrasound enhancing agents) of an apical four-chamber view demonstrating severely reduced LV systolic function and LV apical hypokinesis with an associated LV apical thrombus.
Video 4. CMR CINE sequence two-chamber ventricular long axis demonstrating an apical mass, normal left ventricular size with severe systolic dysfunction (LVEF) 21.79%, severe hypokinesis of the anterior wall and akinesis of the apex and apical lateral wall.
Video 5. CMR CINE sequence four-chamber short axis demonstrating the apical mass and severe hypokinesis of the anterior wall and akinesis of the apex and apical lateral wall. Apical wall appears mildly aneurysmal.
Video 6. CMR two-chamber short axis first-pass perfusion imaging sequences with injection of gadolinium demonstrating absence of uptake on first-pass perfusion of the mid anterior wall consistent with infarct pattern.
Video 7. CMR two-chamber short axis first-pass perfusion imaging sequences with injection of gadolinium demonstrating absence of uptake on first-pass perfusion of the apical and mid lateral wall consistent with infarct pattern. Apical mass is also demonstrated with absence of uptake on first-pass perfusion indicating a mass without native blood supply consistent with a thrombus.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The informed consent was obtained.
Author Contributions
All authors reviewed the literature and helped write the manuscript. Sohiub N. Assaf, MD, Abdallah Assaf, MD, John Taylor, DO, and Jeffery Johnson, MD performed critical revisions of article and approved the final version of manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Takeuchi K, Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve. 1957;9:37-43.
- Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360(12):1226-1237.
doi - Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100(2 Suppl Pediatrics):142-149.
doi - Parmar RC, Bavdekar SB, Muranjan MN, Limaye U. Chorea: an unusual presenting feature in pediatric Moyamoya disease. Indian Pediatr. 2000;37(9):1005-1009.
- Czabanka M, Pena-Tapia P, Schubert GA, Woitzik J, Vajkoczy P, Schmiedek P. Characterization of cortical microvascularization in adult moyamoya disease. Stroke. 2008;39(6):1703-1709.
doi - Bang OY, Fujimura M, Kim SK. The Pathophysiology of Moyamoya Disease: An Update. J Stroke. 2016;18(1):12-20.
doi pubmed pmc - Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, Kim DH, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84(5):617-627.
doi pubmed pmc - De Virgilio A, Greco A, Magliulo G, Gallo A, Ruoppolo G, Conte M, Martellucci S, et al. Polyarteritis nodosa: A contemporary overview. Autoimmun Rev. 2016;15(6):564-570.
doi - Jee TK, Yeon JY, Kim SM, Bang OY, Kim JS, Hong SC. Prospective screening of extracranial systemic arteriopathy in young adults with moyamoya disease. J Am Heart Assoc. 2020;9(19):e016670.
doi pubmed pmc - Kaczorowska M, Jozwiak S, Litwin M, Kmiec T, Kuczynski D, Jurkiewicz E, Koscierza I. [Moyamoya disease associated with stenosis of extracranial arteries: a case report and review of the literature]. Neurol Neurochir Pol. 2005;39(3):242-246.
- Calvet D, Touze E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010;121(14):1623-1629.
doi - Witt BJ, Gami AS, Ballman KV, Brown RD, Jr., Meverden RA, Jacobsen SJ, Roger VL. The incidence of ischemic stroke in chronic heart failure: a meta-analysis. J Card Fail. 2007;13(6):489-496.
doi - Mendes Fde S, Atie J, Garcia MI, Gripp Ede A, Sousa AS, Feijo LA, Xavier SS. Atrial fibrillation in decompensated heart failure: associated factors and in-hospital outcome. Arq Bras Cardiol. 2014;103(4):315-322.
doi pubmed pmc - Komiyama M, Nishikawa M, Yasui T, Otsuka M, Haze K. Moyamoya disease and coronary artery disease—case report. Neurol Med Chir (Tokyo). 2001;41(1):37-41.
doi - Suzui H, Hoshimaru M, Takahashi JA, Kikuchi H, Fukumoto M, Ohta M, Itoh N, et al. Immunohistochemical reactions for fibroblast growth factor receptor in arteries of patients with moyamoya disease. Neurosurgery. 1994;35(1):20-24; discussion 24-25.
doi - Furuta K, Homma T, Yoshioka J, Tamura Y, Hirabayashi H, Sasaki Y, Kawa S, et al. [A case of moyamoya disease associated with stenosis of the right coronary artery, sick sinus syndrome and hypertrophic cardiomyopathy]. Kokyu To Junkan. 1985;33(11):1401-1406.
- Shiratori K, Akasaka T, Asaka T, Shibuya Y, Suzuki K, Yoshida K, Koizumi K, et al. A case of a young woman with occlusion of the circle of Willis (moyamoya disease) presenting angina pectoris. Jpn Circ J. 1985;49(Suppl):112.
- Sakamoto K, Matsumoto M, Miyamoto N, Kounaka S, Okimoto T, Dohi Y. A case of angina pectoris with moyamoya-like cerebro-vascular lesions. Jpn Circ J. 1987;51(Suppl):239.
- Saito K, Hirano H, Katsumata U, Sato K, Matsumoto N, Onodera Y, Miura T, et al. A case presenting an intracranial hemorrhage due to moyamoya disease in association with stenosis of the anterior descending branch of the left coronary artery. Jpn Circ J. 1987;51(Suppl):24.
- Tateno M, Oono T, Ooshima N, Kato N, Tsukada H, Iwasaki T, Sakurai F, et al. A case of a young woman with moyamoya disease presenting angina pectoris. Nippon Naika Gakkai Zasshi. 1988;77:100.
- Suzuki K, Kawarada U, Takeuchi N, Mine T, Ooneda G, Takatama M. An autopsy case of moyamoya disease died of acute heart failure. Gunma Igaku. 1989;50:211-214.
- Tokunaga Y, Toyoda K, Ago T, Ibayashi S, Usui M, Fujishima M. [Systemic vascular change associated with moyamoya-like cerebrovascular disease and microvascular coronary artery disease]. Rinsho Shinkeigaku. 1996;36(2):318-322.
- Akasaki T, Kagiyama S, Omae T, Ohya Y, Ibayashi S, Abe I, Fujishima M. Asymptomatic moyamoya disease associated with coronary and renal artery stenoses—a case report. Jpn Circ J. 1998;62(2):136-138.
doi - Ikeda U, Fujikawa H, Shimada K. Variant angina pectoris associated with moyamoya disease. Lancet. 1998;351(9097):183-184.
doi - Ahn YK, Jeong MH, Bom HS, Park JC, Kim JK, Chung DJ, Chung MY, et al. Myocardial infarction with Moyamoya disease and pituitary gigantism in a young female patient. Jpn Circ J. 1999;63(8):644-648.
doi - St Goar FG, Gominak SC, Potkin BN. Bilateral aortoostial coronary artery disease: moyamoya of the heart? Am J Cardiol. 1999;83(8):1296-1299.A1210.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


