| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 4, April 2023, pages 118-123
Tocilizumab Treatment for Takayasu Arteritis in Pregnancy: A Case Report With Positive Maternal and Neonatal Outcomes
Kota Sugisakia, b, Michihiro Sakauchia
aDepartment of Rheumatology, Japanese Red Cross Mito Hospital, Ibaraki, Japan
bCorresponding Author: Kota Sugisaki, Department of Rheumatology, Japanese Red Cross Mito Hospital, Mito-city, Ibaraki 310-0011, Japan
Manuscript submitted March 23, 2023, accepted April 21, 2023, published online April 30, 2023
Short title: TCZ for Takayasu Arteritis in Pregnancy
doi: https://doi.org/10.14740/jmc4083
| Abstract | ▴Top |
Takayasu arteritis (TAK) is a rare vasculitis that often affects young women of childbearing age, and its management during pregnancy poses unique challenges. Limited data exist regarding the safety and efficacy of tocilizumab (TCZ), an interleukin-6 receptor antagonist, in the treatment of TAK during pregnancy. This case report presents a unique and valuable insight into the use of TCZ in pregnant patients with TAK. We report an 18-year-old female patient with TAK who was treated with TCZ during two pregnancies, resulting in positive maternal and neonatal outcomes. However, a newly identified descending aortic aneurysm was noted after the second delivery, highlighting the importance of careful monitoring of vascular lesions in patients with TAK receiving TCZ. Our findings suggest that TCZ has a high safety profile for both the mother and fetus; however, further research and close monitoring are essential for its use in pregnant patients with TAK.
Keywords: Takayasu arteritis; Tocilizumab; Pregnancy; Drug safety; Tolerability; Vascular lesions; Monitoring
| Introduction | ▴Top |
Rheumatic diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus, often affect women of childbearing age, posing challenges for clinicians when patients wish to conceive or when an unplanned pregnancy occurs [1]. In such cases, therapeutic strategies need to be re-evaluated to ensure the safety of both the mother and fetus. Over the years, clinical experience has identified some medications that are considered relatively safe during pregnancy, and they can be recommended when a change in treatment is required [2]. Takayasu arteritis (TAK) is a large-vessel vasculitis of unknown etiology that primarily affects young women, similar to other rheumatic diseases; however, because of its rarity, its management during pregnancy remains poorly studied. Consequently, the available evidence is limited and often relies on case reports or small case series, with conservative steroidal agents, such as prednisolone (PSL), remaining the mainstay of treatment during pregnancy [3].
This case report presents a young female patient with TAK who was receiving maintenance therapy with tocilizumab (TCZ), an interleukin-6 receptor antagonist. The patient became pregnant twice during treatment and, after careful consultation with her doctor, chose to continue TCZ therapy during her pregnancies, resulting in the safe delivery of healthy newborns on both occasions. Notably, the second pregnancy and delivery occurred during TCZ monotherapy. However, a new descending aortic aneurysm was discovered after the second delivery, despite the patient being asymptomatic. This case highlights the need to discuss the safety of TCZ in pregnancy and the importance of follow-up for vascular lesions, even in asymptomatic patients.
| Case Report | ▴Top |
Investigations
An 18-year-old female patient presented to a nearby neurosurgical hospital in March 2014 with intermittent headaches and general fatigue in her upper limbs that had been present since childhood. She had no significant medical history aside from the aforementioned symptoms and no notable family history. Magnetic resonance imaging of the head revealed a suspected hypoplasia of the left common carotid artery. TAK was suspected, and the patient was referred to our department in April 2014.
On her initial visit at our hospital, the patient exhibited a low-grade fever of 37.4 °C. Her blood pressure was 142/82 mm Hg in the right arm and 124/86 mm Hg in the left arm, with a left-right difference. Her heart sounds were clear, and her pulse rate was 86 beats/min, with a regular sinus rhythm. The bilateral radial arterial pulsations were weakly palpable and did not differ between sides. A right-dominant bruit was heard bilaterally in the neck and supraclavicular fossa. No bruits were heard in the abdomen. Laboratory data on admission were as follows: increased C-reactive protein (CRP) level, 4.11 (normal, < 0.3) mg/dL; erythrocyte sedimentation rate, 32 (normal, < 20) mm/h; white blood cell count, 9,190 (normal, 3,000 - 8,000)/mm3; hemoglobin level, 12.4 (normal, 10 - 14) g/dL; platelet count, 30.4 (normal, 15 - 40) × 104/mm3; and normal levels of hemolytic complement, 45.5 (normal, 30 - 50) IU/mL, and C3, 88.2 (normal, 86 - 160) mg/dL. Serum antinuclear antibodies were negative on indirect immunofluorescence testing using Hep2 cell substrates. No significant hepatorenal damage was observed. No abnormal urinalysis findings were observed. Transthoracic echocardiography did not reveal aortic regurgitation.
Diagnosis
Whole-body computed tomography (CT) and CT angiography were performed to visualize the anatomy of the blood vessels, assess the extent of arterial wall thickening, and identify potential stenosis or occlusions. The imaging revealed stenosis of the bilateral common carotid, subclavian, and vertebral arteries (Fig. 1a-c). No abnormalities were observed in the ascending aorta, descending aorta, or renal arteries. Additionally, positron emission tomography (PET)/CT was conducted to provide functional information regarding vascular inflammation by detecting increased metabolic activity in the affected arterial wall, indicative of active arteritis. PET/CT showed 18F-fluorodeoxyglucose (FDG) accumulation in both the common carotid arteries and descending thoracic aorta (Fig. 1d, e). Based on these findings, the patient was diagnosed with TAK and admitted to our department in late April.
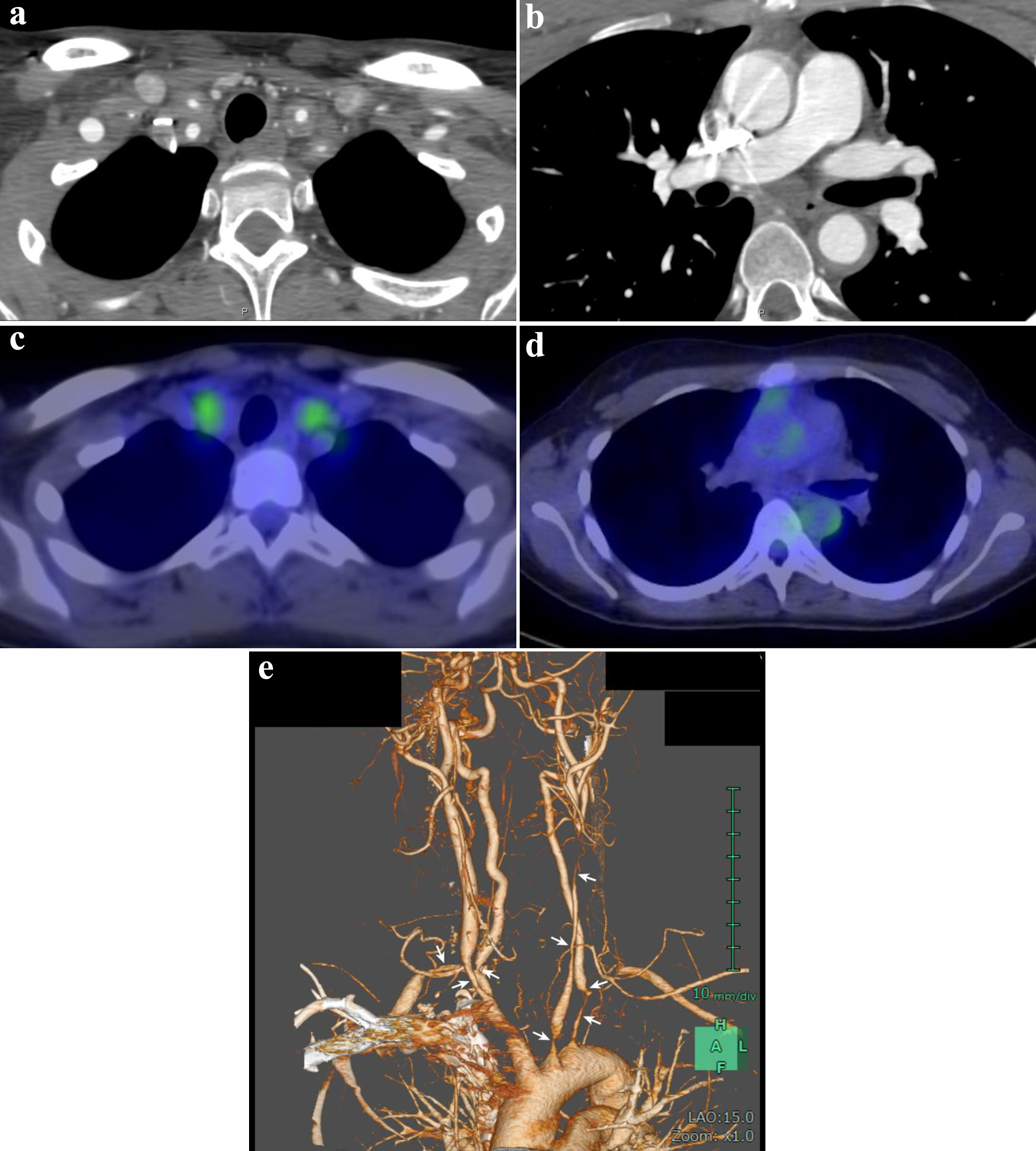 Click for large image | Figure 1. Contrast-enhanced computed tomography (CT) (a and b), positron emission tomography/CT (c and d), and CT angiography (e) performed prior to the treatment. (a) Marked wall thickness and luminal constriction of the bilateral common carotid and subclavian arteries. (b) The descending aorta shows wall thickening but no apparent luminal stenosis or dilation. (c) 18F-Fluorodeoxyglucose accumulation is observed in the bilateral common carotid arteries. (d) 18F-Fluorodeoxyglucose accumulation is observed in the ascending and descending aortas. (e) Stenosis is discernible in the bilateral carotid, subclavian, and vertebral arteries (arrows). |
Treatment
The patient was treated with PSL at a dose of 30 mg/day. After the start of treatment, her headache quickly subsided, and her fever resolved and did not recur. No serious adverse reactions associated with PSL treatment were observed; however, an elevated blood pressure was noted. Her systolic blood pressure, measured in the right upper arm, increased to approximately 150 - 160 mm Hg, and her diastolic blood pressure also increased accordingly. Consequently, amlodipine was administered at a dose of 5 mg/day to manage hypertension, and her blood pressure subsequently decreased to 130 mm Hg. Antiplatelet agents were considered; however, the patient did not wish to take them because PSL alone was sufficient to alleviate her symptoms. The patient was discharged in early May 2014 and continued outpatient treatment. Her CRP level was negative at 0.2 mg/dL at discharge. When the PSL dose was gradually reduced to 15 mg/day, a mild increase in the CRP level was observed without any symptoms. Although tacrolimus (TAC) was administered at a dose of 2 mg/day, the patient’s CRP level remained positive. When the PSL dose was reduced to 11 mg/day, her headache flared with no accompanying increase in blood pressure. After deciding that more aggressive control of inflammation was necessary, subcutaneous TCZ injection (162 mg weekly) was initiated in January 2018. The patient’s symptoms resolved, and her CRP level immediately became negative, allowing for further reduction of PSL. When the PSL dose was tapered to 5 mg/day, amlodipine was discontinued. However, the patient’s blood pressure remained stable at 120 mm Hg. It is worth noting that although TAC can cause hypertension, the patient’s blood pressure was well controlled during the treatment.
Follow-up and outcomes
In August 2018, the patient discovered that she was pregnant for the first time. At that time, the patient was receiving treatment with 2.5 mg/day of PSL, 2 mg/day of TAC, and the same TCZ dose. The physician informed her that it had been demonstrated that TCZ did not appear to exert a significant impact on the progression of pregnancy or the outcome of the child with rheumatic disorders, particularly in patients with RA [4]. However, it was unknown whether the same could be said for TAK cases. Additionally, the physician mentioned that there were scarce reports of continuous TCZ use during pregnancy. He also explained that empirically, it was considered reasonable to discontinue TCZ therapy during pregnancy and treat it with PSL, which was known to be generally safe for the fetus; although an increase in PSL may be necessary to control TAK, it can still result in adverse effects such as the risk of intrauterine growth restriction, preeclampsia, and preterm delivery. After carefully considering the physician’s explanation, the patient decided to continue with TCZ during her pregnancy because she felt well after initiating TCZ and was apprehensive about the increased side effects of PSL. The patient was closely monitored throughout her pregnancy, with no alterations to the treatment plan. TAK activity was monitored during pregnancy by checking for the recurrence of symptoms such as headache and upper limb fatigue, as well as maintaining a negative CRP level during monthly outpatient visits. During the pre-delivery period, there were no major issues with fetal development or symptoms, suggesting enhanced TAK activity. In mid-April 2019 (at a gestational age of 40 weeks), the patient was admitted to the hospital because of labor pain and was diagnosed with gestational hypertension syndrome after her blood pressure in the right arm increased to > 160 mm Hg. An emergency cesarean section was performed, and a healthy male infant weighing 3,182 g was delivered (Apgar score, 3/9). TCZ was temporarily discontinued as one dose only after emergency cesarean section. Her postoperative course was uneventful, and her blood pressure decreased to 130 mm Hg without antihypertensive medication. She was discharged with her infant at the end of April.
Because the patient presented with no specific symptoms and her CRP level remained within the normal range, PSL was discontinued in May 2020, and TAC was discontinued in September of the same year. TCZ monotherapy was continued without symptom recurrence or elevated CRP levels. In July 2021, the patient became pregnant again. Similar to her previous pregnancy, there were no major complications in either the mother or child, and TCZ monotherapy was continued. A scheduled cesarean section was performed in late February 2022 at a gestational age of 37 weeks, resulting in the delivery of a healthy female infant weighing 2,865 g. Similar to her previous pregnancy, TCZ was temporarily discontinued during the perioperative period of the cesarean section in the second pregnancy and was limited to a single dose. The patient’s blood pressure had not increased during the previous delivery period. The patient was discharged with her infant in early March. Throughout both pregnancies, there were no notable infections reported for the mother or newborns during their first 6 months of life.
On discharge, TCZ monotherapy was continued. Despite presenting with no specific symptoms and no alterations in the CRP levels, CT angiography performed in September 2022 showed the formation of an aneurysm measuring 29 mm in diameter in the descending aorta despite improvement in the wall thickening of both the common carotid and subclavian arteries (Fig. 2). In the follow-up CT angiography performed in March 2023, no significant changes were observed in the morphology of the descending aortic aneurysm, and there was no worsening of wall thickening in either the common carotid or subclavian arteries.
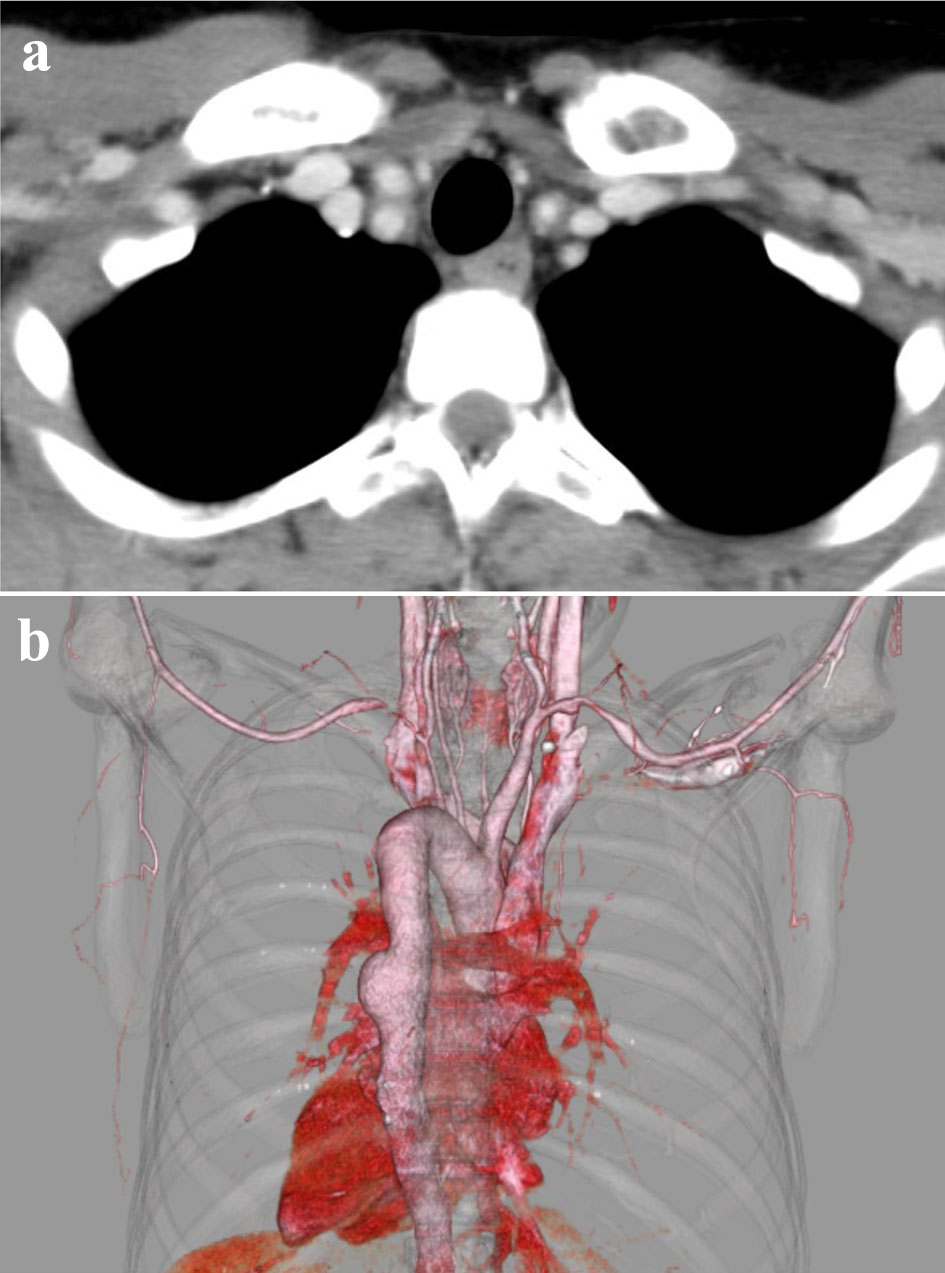 Click for large image | Figure 2. Contrast-enhanced computed tomography (CT) (a) and CT angiography (b) performed in September 2022. (a) The wall thickening of the bilateral common carotid and subclavian arteries has improved. (b) The formation of an aneurysm 29 mm in diameter is observed in the descending aorta. |
| Discussion | ▴Top |
TCZ was originally developed as an intravenous formulation for RA treatment. Subsequently, subcutaneous injection formulations were introduced, and in Japan, subcutaneous injection of TCZ (162 mg once weekly) for the treatment of TAK was approved for health insurance coverage in 2017. According to the Japanese guidelines for the management of vasculitis syndromes, drugs such as methotrexate, azathioprine, mycophenolate mofetil, calcineurin inhibitors, and tumor necrosis factor inhibitors are listed as treatment options for TAK; however, only steroidal agents and TCZ have a class I recommendation [5]. The ACT-Bridge Study, a post-marketing surveillance study of TCZ, demonstrated that 20% of patients experienced a relapse of disease activity (e.g., fever, neck pain, headache, abnormal imaging findings, elevated inflammatory marker levels) during TCZ treatment, yet a steroid-tapering effect was observed [6]. TCZ, which is prone to flare-ups following steroidal agent reduction, is considered a practical option for the treatment of TAK.
Although Nakajima et al showed that exposure to TCZ at the time of pregnancy diagnosis did not increase the incidence of spontaneous abortion and birth defects, most cases in their study involved RA, and in three-quarters of the cases, TCZ was discontinued by the first trimester of pregnancy. TCZ was continued during pregnancy in only two of 61 cases [4]. The results of this study cannot be directly applied to TAK cases, and no clear conclusions have been reached to date regarding the safety of continued TCZ use during pregnancy. In contrast, two cases have been reported in which TCZ was used continuously during pregnancy in patients with severe TAK who delivered healthy newborns [7]. Furthermore, Moriyama et al reported a very low TCZ concentration in the cord blood (7.6% of maternal serum) in a case of TAK in which TCZ was continued throughout pregnancy [8]. Similar results have been reported for adult-onset Still’s disease [9]. Therefore, we believe that there is little need to categorically reject TCZ administration during pregnancy. Additionally, TCZ for intravenous infusion was approved for coronavirus disease 2019 (COVID-19) pneumonia in 2022, and Jorgensen et al found no serious safety concerns in pregnant women with COVID-19 who received TCZ, despite limited data [10].
CT angiography performed after the patient’s second delivery showed a descending aortic aneurysm that had not been previously identified. Although we believe that there is a strong association between the FDG uptake in the descending aorta observed on the PET/CT at diagnosis and the subsequent discovery of the descending aortic aneurysm, it is not possible for us to determine, within the available information, whether the aneurysm developed before the initiation of TCZ therapy or emerged as a new lesion during treatment. The lack of prior angiography contributes to this limitation in our case report. The patient experienced two pregnancies, which should have been taken into consideration; however, the vascular lesions were not re-evaluated over an extended period. The ACT-Bridge Study found that 50% of the patients who were determined to have relapsed during TCZ treatment developed worse vascular lesions. Other reports have also documented the progression of vascular lesions during TCZ treatment [11-13], including one case in which the patient was asymptomatic but exacerbation was observed on vascular imaging [14]. A review of 28 patients with TAK treated with TCZ for 2 years by Nakaoka et al found that approximately 40% of patients had new or worsening vascular lesions [15]. These findings suggest that the development of vascular lesions is common during TCZ treatment and that periodic angiography is advisable even in the absence of an inflammatory response or symptom flare-ups. On the other hand, in the present case, concurrent with the development of a new descending aortic aneurysm, improvement in the wall thickening of the common carotid and subclavian arteries was also observed. Although a definitive conclusion cannot be reached for the aforementioned reasons, it is probable that TCZ therapy was therapeutically effective for these arteries because the patient’s headache symptoms improved rapidly following the initiation of TCZ therapy and did not recur after the subsequent reduction of the PSL dose.
There is a possibility that the marked hemodynamic changes during pregnancy could have caused damage to weakened vessel walls due to inflammation, ultimately leading to the development or enlargement of aneurysms. Although there has been a report of a case where aneurysms that tend to enlarge during pregnancy were discovered first and subsequently diagnosed as TAK [16], it is rare to find reports of new or existing aneurysms enlarging during the course of pregnancy in patients who have already been diagnosed with TAK. In our opinion, it is possible that during pregnancy, blood pressure management by obstetricians is carefully performed, thereby reducing the pressure load that would rapidly enlarge aneurysms.
In conclusion, our case report suggests that TCZ treatment can be continued in patients with TAK during pregnancy, although caution is necessary. However, inadequate planning of vascular lesion evaluation limited our ability to assess the efficacy of TCZ in preventing the development of vascular lesions in this patient. Our findings underscore the importance of regular vascular image evaluations, not only for patients with TAK in general but also particularly for women planning to conceive, to ensure the best outcomes for both the mother and fetus.
Learning points
Although our experience is limited, TCZ administration during pregnancy appears to have a high safety profile for both the mother and fetus. In cases of TAK, planned imaging evaluation of vascular lesions is necessary to accurately assess treatment efficacy, even in asymptomatic patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The patient’s written informed consent for publication was obtained.
Author Contributions
Kota Sugisaki, MD, and Michihiro Sakauchi, MD: diagnosis and management of the patient; discussion, writing, and drafting of the case; and final approval of the case report.
Data Availability
The authors declare that the data supporting the findings of this study are available in this article.
Abbreviations
CRP: C-reactive protein; CT: computed tomography; PET: positron emission tomography; PSL: prednisolone; RA: rheumatoid arthritis; TAC: tacrolimus; TAK: Takayasu arteritis; TCZ: tocilizumab
| References | ▴Top |
- Gotestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, da Silva J, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795-810.
doi pubmed - Ostensen M, Forger F. Management of RA medications in pregnant patients. Nat Rev Rheumatol. 2009;5(7):382-390.
doi pubmed - Machen L, Clowse ME. Vasculitis and pregnancy. Rheum Dis Clin North Am. 2017;43(2):239-247.
doi pubmed - Nakajima K, Watanabe O, Mochizuki M, Nakasone A, Ishizuka N, Murashima A. Pregnancy outcomes after exposure to tocilizumab: A retrospective analysis of 61 patients in Japan. Mod Rheumatol. 2016;26(5):667-671.
doi pubmed pmc - Isobe M, Amano K, Arimura Y, Ishizu A, Ito S, Kaname S, Kobayashi S, et al. JCS 2017 guideline on management of vasculitis syndrome - digest version. Circ J. 2020;84(2):299-359.
doi pubmed - Harigai M, Miyamae T, Hashimoto H, Yoshida A, Yamashita K, Nakaoka Y. A multicentre, large-scale, observational study of tocilizumab in patients with Takayasu arteritis in Japan: the ACT-Bridge study. Mod Rheumatol. 2022;3:roac099.
doi pubmed - Cruz-Machado AR, Andrade Silva L, Barreira SC, Veiga A, Ponte C, Pinto L, Macieira C, et al. Tocilizumab throughout pregnancy in two patients with severe Takayasu's arteritis. Acta Reumatol Port. 2021;46(2):193-195.
pubmed - Moriyama M, Wada Y, Minamoto T, Kondo M, Honda M, Murakawa Y. Unexpectedly lower proportion of placental transferred tocilizumab relative to whole immunoglobulin G: a case report. Scand J Rheumatol. 2020;49(2):165-166.
doi pubmed - Saito J, Yakuwa N, Kaneko K, Takai C, Goto M, Nakajima K, Yamatani A, et al. Tocilizumab during pregnancy and lactation: drug levels in maternal serum, cord blood, breast milk and infant serum. Rheumatology (Oxford). 2019;58(8):1505-1507.
doi pubmed - Jorgensen SCJ, Lapinsky SE. Tocilizumab for coronavirus disease 2019 in pregnancy and lactation: a narrative review. Clin Microbiol Infect. 2022;28(1):51-57.
doi pubmed pmc - Bredemeier M, Rocha CM, Barbosa MV, Pitrez EH. One-year clinical and radiological evolution of a patient with refractory Takayasu's arteritis under treatment with tocilizumab. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S98-S100.
pubmed - Liebling EJ, Peterson R, Victoria T, Burnham JM. Aortic ulceration in a tocilizumab-treated patient with Takayasu arteritis. Ann Rheum Dis. 2019;78(10):e116.
doi pubmed - Sanchez-Alvarez C, Koster M, Duarte-Garcia A, Warrington KJ. Disease progression of Takayasu arteritis in two patients treated with tocilizumab. Ann Rheum Dis. 2020;79(2):e21.
doi pubmed - Muratore F, Salvarani C. Aortic dilatation in a patient with Takayasu arteritis treated with tocilizumab. Ann Rheum Dis. 2021;80(7):e121.
doi pubmed - Nakaoka Y, Yanagawa M, Hata A, Yamashita K, Okada N, Yamakido S, Hayashi H, et al. Vascular imaging of patients with refractory Takayasu arteritis treated with tocilizumab: post hoc analysis of a randomized controlled trial. Rheumatology (Oxford). 2022;61(6):2360-2368.
doi pubmed pmc - Bartczak-Rutkowska A, Trojnarska O, Cieplucha A, Janus M, Jemielity M, Lesiak M. Enlarging aneurysm of the ascending aorta in a pregnant woman with Takayasu arteritis. Kardiol Pol. 2020;78(1):82-83.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


