| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 8, August 2023, pages 299-306
A Changing Anti-Neutrophil Cytoplasmic Antibody Profile in a Patient With a Diagnosis of Eosinophilic Granulomatosis With Polyangiitis
Yusuke Jinnoa, Yutaka Kozua, b , Hisato Hiranumaa, Shuichiro Maruokaa, Yasuhiro Gona
aDivision of Respiratory Medicine, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan
bCorresponding Author: Yutaka Kozu, Division of Respiratory Medicine, Department of Internal Medicine, Nihon University School of Medicine, Tokyo 173-8610, Japan
Manuscript submitted April 3, 2023, accepted August 25, 2023, published online August 28, 2023
Short title: ANCA Changes in EGPA
doi: https://doi.org/10.14740/jmc4088
| Abstract | ▴Top |
This report describes a hitherto unique case of eosinophilic granulomatosis with polyangiitis (EGPA), a subtype of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. The patient was an 81-year-old man whose clinical course involved notable changes in the ANCA profile, specifically a transition from positive proteinase 3 (PR3)-ANCA to myeloperoxidase (MPO)-ANCA, followed by simultaneous positivity for both. The patient’s medical history included bronchial asthma, allergic rhinitis, sinusitis, and multiple comorbidities. Despite being initially PR3-ANCA-positive, subsequent admissions demonstrated MPO-ANCA positivity along with eosinophilic manifestations, highlighting the complexity of diagnosis of EGPA. Diagnostic evaluation included imaging, serological markers, and clinical symptoms, which collectively supported the classification of EGPA. Notably, this case challenges the conventional diagnostic paradigms and emphasizes the evolving nature of ANCA profiles in vasculitis. The shift in ANCA profile prompted a reevaluation of the patient’s diagnosis and treatment strategy. This case underscores the importance of considering fluctuations in ANCA in patients with a diagnosis of EGPA, management decisions, and potential implications for disease progression. Further research is warranted to elucidate the mechanisms underlying changes in ANCA and their clinical significance in vasculitis.
Keywords: Antineutrophil cytoplasmic antibody; Eosinophilic granulomatosis with polyangiitis; Vasculitis
| Introduction | ▴Top |
Small-vessel vasculitis involves arterioles, capillaries, and venules, and sometimes small arteries. One type of small-vessel vasculitis affects the entire body and was originally known as Churg-Strauss syndrome [1] but underwent a name change to eosinophilic granulomatosis with polyangiitis (EGPA) after its classification at the Chapel Hill Consensus Conference in 2012 [2]. In the Japanese population, EGPA has a prevalence of 1.78 per 100,000, is usually observed in those aged 30 - 60 years, and has a male-to-female ratio of 1:2 [3]. EGPA causes necrotizing vasculitis and granulomatous inflammation and is composed of three phases: 1) a prodromal stage characterized by allergic conditions such as bronchial asthma and allergic rhinitis; 2) a period in which the number of peripheral eosinophils increases markedly, and local infiltration such as eosinophilic pneumonia is observed; and 3) a period during which symptoms of multi-organ vasculitis appear. However, it is important to note that the clinical presentation of EGPA may not always strictly follow this triphasic pattern. Furthermore, the extent of vasculitis involvement may vary, ranging from multifocal to organ-limited manifestations. The heterogeneous nature of EGPA underscores the complexity of its pathogenesis and the challenges in its diagnosis and management. EGPA is classified using the American College of Rheumatology criteria [4]. It is important to note that unlike diagnostic criteria, classification criteria are intended to provide a standardized framework for research and clinical studies rather than strict guidelines for diagnosis and treatment of individual patients.
Recent studies have provided valuable insights into the pathophysiology of EGPA and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Notably, research by Haruki et al in 2022 visualized the dynamic behavior of eosinophils in EGPA, highlighting their role in the disease process [5]. They also elucidated the contribution of neutrophil-associated vascular damage in ANCA-associated vasculitis, further enriching our understanding of these complex conditions [6]. Incorporating these recent findings into our understanding, this report aims to contribute to the growing body of knowledge regarding the clinical and immunological features of EGPA.
Here we report a case in which specific changes in PR3-ANCA and myeloperoxidase (MPO)-ANCA were involved in the development of EGPA. The case may help shed light on the serologies involved. Changes in ANCA are deeply involved in the pathology of EGPA.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of The Nihon University Hospital (approval number RK130412-19). Written informed consent was obtained from the patient for publication of this case report.
| Case Report | ▴Top |
Investigations
An 81-year-old man complained of fever and dyspnea. He had a history of allergic rhinitis and had undergone surgery for sinusitis at the age of 20 years. Despite recurrent symptoms of sinusitis, he had not sought further treatment. He had developed asthma in adulthood, but the symptoms were stable and did not require treatment. He also had lumbar spinal stenosis, prostatic hyperplasia, sinusitis, hypertension, hypothyroidism, iron deficiency anemia, angina, and chronic heart failure. He reported no drug or food allergies. His medications included diltiazem 100 mg/day, neurotropin 16 U/day, mecobalamin 1.5 mg/day, mirabegron 50 mg/day, pregabalin 50 mg/day, teprenone 150 mg/day, iron supplementation of 105 mg/day, and febuxostat 10 mg/day (the initial treatment regimen for gout in Japan). At the time of the third admission, a blood test had shown elevated C-reactive protein and eosinophil levels and a positive PR3-ANCA result. The authors examined ANCA using the chemiluminescent enzyme immunoassay method, with the reference values for both PR3-ANCA and MPO-ANCA being below 3.5 U/mL. In terms of cardiac markers, the N-terminal pro-B-type natriuretic peptide level was mildly elevated at 251 ng/L. However, cardiac ultrasound did not reveal any evidence of heart failure. Ground-glass opacities were observed on chest computed tomography (CT). However, 2 weeks prior to the present admission, fever, left precordial pain, and dyspnea were noted, and the patient was hospitalized for further examination and treatment of suspected pneumonia and heart failure.
The patient’s height was 160 cm, his bodyweight was 69 kg, and his body mass index was 26.9. His vital signs were as follows: blood pressure 115/60 mm Hg, heart rate 62 bpm, respiratory rate 21 bpm, body temperature 36.9 °C, and oxygen saturation 94% (nasal tube 3 L/min). Chest auscultation revealed coarse crackles in the lower left lung field and no signs of wheeze or a heart murmur. His abdomen was flat and non-tender. There was no lower leg edema; however, a rash was observed on the lower extremities. There were no neurological findings.
A transition of ANCA serologies occurred during these two hospitalizations. At the first hospital admission, PR3-ANCA was positive; however, during outpatient follow-up, PR3-ANCA became negative and MPO-ANCA turned positive. After 9 months, there was worsening of the ground-glass opacities on chest CT, and PR3-ANCA turned positive again. Three months after the MPO-ANCA and PR3-ANCA became positive, the patient was hospitalized a further time.
Diagnosis
Laboratory data were collected and are summarized in Table 1. Hematological findings included an increased eosinophil count (435/µL) and decreased renal function (aspartate transaminase 49 IU/L, creatinine 1.38 mg/dL). The N-terminal pro-B-type natriuretic peptide and Krebs von den Lungen-6 levels were within the reference range, and there were no other findings suggestive of systemic autoimmune disease. Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus grew on sputum culture. Subsequently, his C-reactive protein levels decreased steadily. Nevertheless, the two ANCA serologies and eosinophil counts increased, the pleural effusion did not improve, and his symptoms worsened. Thoracocentesis was performed; the pleural effusion was exudative with lymphocytes. The number of eosinophils increased significantly to 5,635/µL, and both types of ANCA increased. The patient met Lanham’s criteria and was diagnosed with EGPA.
 Click to view | Table 1. Summary of Laboratory Findings |
The urinalysis findings were normal. The immunoserologic test results suggested systemic autoimmune disease and were negative for procalcitonin, β-D glucan, Aspergillus antigen, Mycoplasma antibody, urine Legionella antibody, and influenza A/B antigen. The arterial blood gas test was performed with O2 at 3 L/min and revealed a pH of 7.443, PaO2 of 51.9 mm Hg, PaCO2 of 31.4 mm Hg, HCO3- of 21.2 mEq/L, and SaO2 of 87.2%. P. aeruginosa and methicillin-susceptible S. aureus were detected in the sputum.
On admission, radiographs revealed right middle and lower lung interlobar infiltrates, a dull right costophrenic angle, and a light shadow in the left middle and lower lung fields. Interlobar thickening and pleural effusion were seen (Fig. 1). A biopsy was performed of the skin lesions, including ulcerative lesions, erythema, and purpura on the front of the left ankle joint. Pathology revealed edematous blood vessel walls with lymphocytic and histiocytic infiltration as well as fibrinoid necrosis around the blood vessels; these findings are consistent with vasculitis, absence of eosinophilic infiltration (Fig. 2).
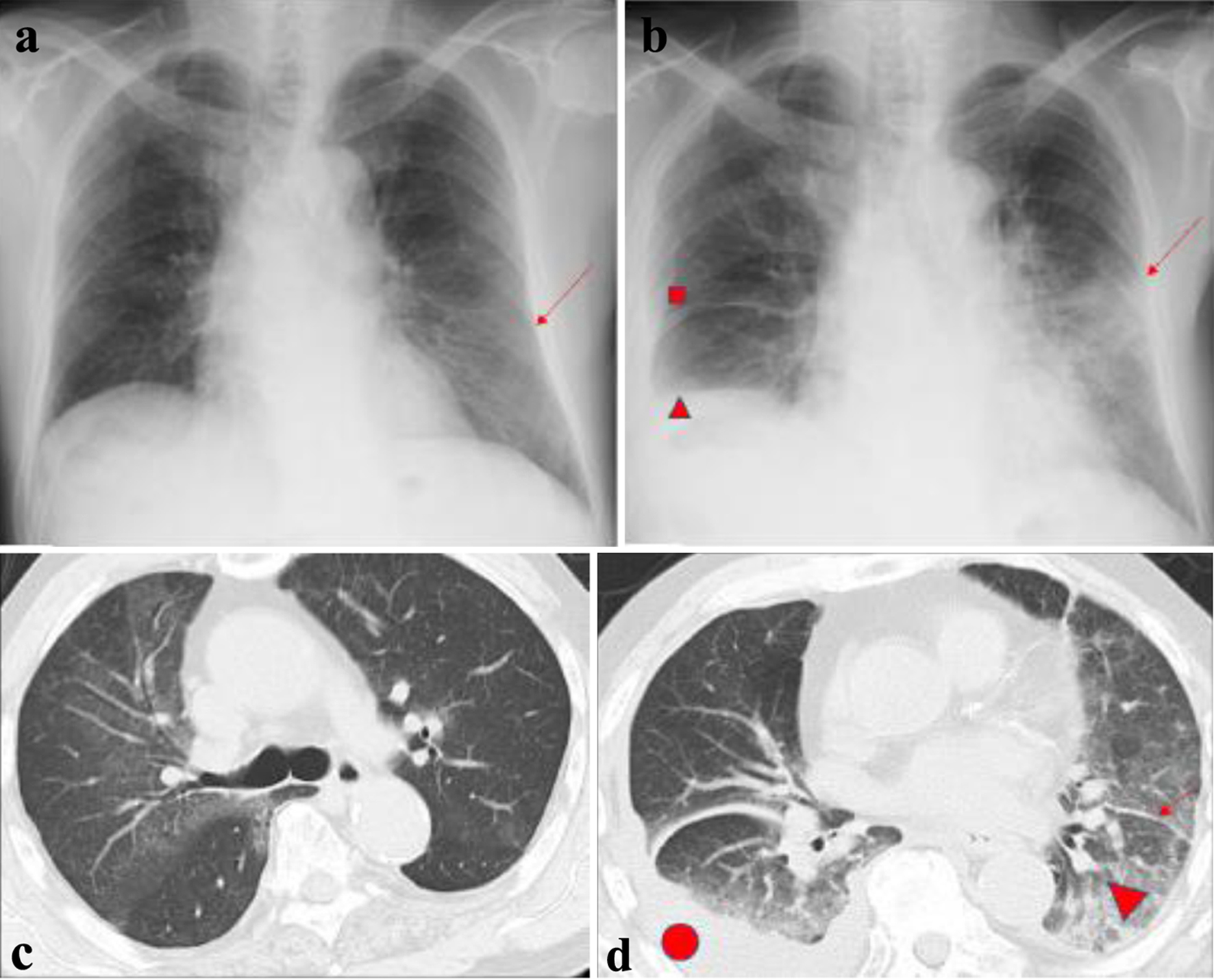 Click for large image | Figure 1. Chest radiographs and computed tomography scans. (a) First admission (arrow indicates ground-glass opacity in the lower left lung field. (b) Third admission (■ indicates interlobar thickening in the right middle lung field; ▲ indicates a dull right costophrenic angle; the arrow indicates consolidation in the left middle and lower lung field). (c) First admission. (d) Third admission (• indicates pleural effusion; ▲ indicates ground-glass opacity; arrow indicates thickness between leaflets). |
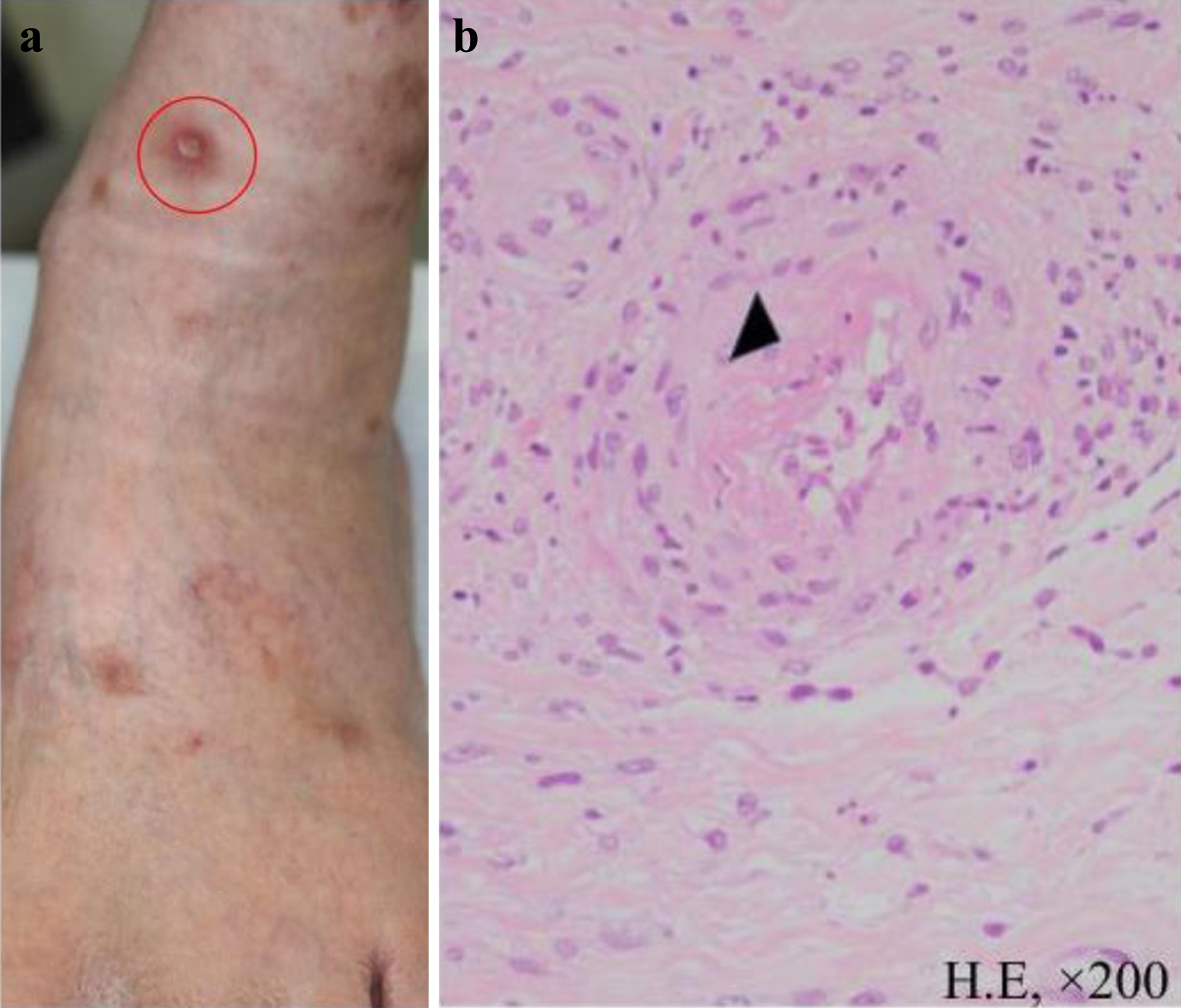 Click for large image | Figure 2. Skin biopsy. (a) Ulcerative lesions, erythema, and purpura were observed on the anterior aspect of the left ankle joint. A biopsy was performed on the ulcerative lesion indicated by the red circle. (b) Pathologic findings (hematoxylin and eosin staining, × 200). The biopsy specimen showed infiltration of lymphocytes and histiocytes with fibrinoid necrosis around blood vessels (indicated by ▲) consistent with vasculitis. |
Treatment
Although the patient had been hospitalized and treated previously, he was treated with antibiotics because bacterial infection was thought to have triggered the pneumonia. Although the possibility of drug-induced eosinophilia cannot be ruled out, the drug was frequently used for a long time and there were no problems even after continued use, so it was excluded as the cause. He was started on prednisolone 30 mg/day. Immediately thereafter, his eosinophil count reduced to 0, his ANCA levels declined, and his symptoms improved. No relapse was observed even when the dose of prednisolone was gradually reduced to 17.5 mg/day; he was discharged 53 days after admission (Table 2).
 Click to view | Table 2. Clinical Course |
Follow-up and outcomes
After discharge, the patient was treated on an outpatient basis while weaning the dosage of steroids. His progress was favorable. In view of the patient’s advanced age and the fact that his condition was well controlled with low-dose steroids alone, we opted to add mepolizumab, which has fewer adverse effects, to his treatment regimen. This decision was made in view of the patient’s age and the desire to minimize the potential risk of adverse events. The patient’s Birmingham Vasculitis Activity Score, which was 15 points at the time of admission, had decreased to 0 points by the time of discharge, and he was reported to be in good condition.
| Discussion | ▴Top |
Our patient’s PR3-ANCA and MPO-ANCA showed changes over time that are hitherto unique. His hospitalizations were associated with acute respiratory failure following infection, followed by increased eosinophil counts. However, imaging findings at the time of the second admission showed a change in the ground-glass opacities, and pleural effusion was noted. Antimicrobial treatment increased the number of eosinophils and exacerbated the pleural effusion (which was exudative, and pleural eosinophil counts were increased at the time of exacerbation). Corticosteroids markedly improved the pleural effusion, ground-glass opacities, and eosinophilia.
It has previously been reported that 2% of patients with vasculitis show changes in PR3-ANCA and MPO-ANCA, as observed in our case [7]. The ANCA test was consistently measured in the same way in our patient. A search of our hospital records for the most recent 5 years identified only two cases of EGPA, and there was no association of ANCA with disease activity. However, it is generally known that there is a relationship between the disease activity of ANCA and EGPA, and ANCA-positive cases with an increase in the number of eosinophils are the most common [8]. There have been no reports of the MPO-ANCA subtype reversing, as in our patient.
More than 10 subtypes of ANCA have been identified. Hyperactivation of neutrophils by ANCA is thought to involve bacterial infection and formation of neutrophil extracellular traps [9]. Different types of ANCA may be positive, as in our case. Moreover, it is possible that another type of ANCA (e.g., bactericidal/permeability-increasing protein-ANCA, which is triggered by P. aeruginosa infection) may be positive on indirect immunofluorescence.
Our patient’s ANCA levels may have decreased before hospitalization because a bacterial infection occurred, causing ANCA to decrease and eosinophil counts to rise. However, when an infection is most active, neutrophils predominate and EGPA is masked. Considering that the ANCA levels decreased, it is possible that the infection subsided and EGPA-related inflammation was reactivated. The PR3 could have been a false positive, but there was a correlation with the transition of symptoms. It is unclear whether PR3 affected the symptoms.
Approximately 30-40% of cases of EGPA are MPO-ANCA-positive and 55-65% are ANCA-negative. The heart is the organ most frequently damaged by vasculitis in EGPA, and the prognosis is said to be poor. Furthermore, 30-45% of patients with EGPA-induced heart damage are ANCA-negative, and thrombosis is attributed to eosinophilic cardiomyopathy and intimal damage in these cases [10]. In our patient, ultrasound was the only cardiac examination performed, and although there is little evidence that EGPA was the cause of his heart failure, this possibility cannot be excluded.
Possible mechanisms for a change in ANCA subtype include abnormalities in the immune system. First, production of ANCA involves immune cells, such as B cells and T cells [11], dysfunction of which may lead to changes in the amount or types of ANCA produced. Second, expression of genes that affect production of ANCA could be regulated by epigenetic changes. For example, changes in DNA methylation may be involved in changes in subtype of ANCA [12]. Third, production of ANCA can be affected by environmental factors [11]. For example, smoking is known to promote production of MPO-ANCA [13]. Hypothyroidism can also cause a positive ANCA. Finally, ANCA may have a high affinity for specific antigens, which is determined by the binding affinity of immunoglobulin. Changes in this affinity may result in changes in the ANCA subtype. These mechanisms are being investigated to further our understanding of changes in ANCA subtype [14].
Within the spectrum of vasculitic disorders, the co-occurrence of ANCA and specific autoantibodies associated with distinct vascular components has garnered attention for its potential immunopathogenic significance. While initially observed in disorders like Goodpasture’s disease, where renal and pulmonary involvement is driven by anti-glomerular basement membrane antibodies, the phenomenon of dual positivity for ANCA and other antibodies has broader implications. This phenomenon challenges the conventional boundaries of antibody specificity, suggesting a more intricate interplay between the different antibody types.
Furthermore, the dynamic nature of antibody profiles extends beyond dual positivity, with shifts in antibody dominance or subtype having been noted over time. This evolving pattern prompts inquiry into the evolving pathogenesis of vasculitic disorders and the potential clinical significance of such shifts. The observed duality and changes in antibody responses raise questions about shared immunopathogenic pathways, potential crosstalk between different antibody-producing cell populations, and the possible influence of one antibody type on the production or response of another.
While initial exploration has focused on Goodpasture’s disease and its dual positivity, this pattern may extend to other vasculitic conditions. Investigating the presence and shifts of multiple antibodies in vasculitic disorders can afford insights into complex immune interactions and the resulting clinical ramifications. In summary, the emerging landscape of dual positivity and antibody changes presents a compelling avenue for future research to unravel the intricate network of immune responses underlying the pathogenesis of vasculitis [15].
The patient’s Five-Factor Score [16] was as follows. High age over 65: the patient’s age was 81 years, meeting this criterion (score 1). Renal insufficiency: information on the patient’s creatinine level was not provided, so it could not be determined if this criterion was met (score 0). Cardiac insufficiency: the patient had a history of angina and chronic heart failure, indicating cardiac involvement (score 1). Severe gastrointestinal involvement: there was no mention of specific gastrointestinal complications such as bowel perforation, bleeding, or pancreatitis in the case report (score 0). Ear, nose, and throat involvement: clinical symptoms consistent with EGPA involving the ear, nose, and throat were confirmed by physical examination and CT (score 1). Based on the definitions and information provided, this patient would have a Five-Factor Score of 3.
EGPA has a high relapse rate. However, we did not use immunosuppressive therapy in our patient because of his advanced age and the possibility of adverse events. After administration of mepolizumab [17, 18], there was no increase in the eosinophil count, even after steroid tapering, and there has been no relapse of symptoms in the year since discharge from hospital. Furthermore, the pleural effusions that persisted from the time of hospital admission through to outpatient follow-up improved after starting mepolizumab [19].
Learning point
This case underscores the significance of monitoring ANCA profiles during the course of EGPA. Shifts from one ANCA serotype to another can influence disease classification and treatment decisions. The complex dynamics of ANCA emphasize the need for multidisciplinary assessments and a comprehensive approach to diagnosis. Our case also highlights the challenges in distinguishing EGPA from other conditions with eosinophilic manifestations. Atypical presentations, like the transition from PR3-ANCA to MPO-ANCA positivity in this patient, emphasize the importance of broader clinical evaluation. The implications of ANCA fluctuations in terms of treatment strategies should not be overlooked. Our case exemplifies how changes in ANCA profile can prompt adjustments in therapeutic decisions, such as considering targeted biologic agents like mepolizumab.
Conclusion
We have encountered a case in which specific changes in PR3-ANCA and MPO-ANCA were involved in the development of EGPA. EGPA is characterized by genetically and clinically different phenotypes. Usually, only one type of ANCA is followed when observing the course of ANCA-associated vasculitis. This is the first report of a case in which changes occurred in a different subtype of ANCA. The case may provide clues regarding the pathology of EGPA and help to establish biomarkers of this disease.
Acknowledgments
The authors would like to express their gratitude to the patient who allowed us to report his case. We are grateful to the medical staff members involved in the patient’s care for their support and assistance.
Financial Disclosure
None to declare.
Conflicts of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report.
Author Contributions
YJ, HH, and YK participated in the patient’s care. YK wrote the first draft of the manuscript. HH, SM, and YG provided oversight and guidance in reviewing and refining the final version of the manuscript. All authors contributed to the conception of the work and revisions, and gave final approval of the version to be published.
Data Availability
Any inquiries regarding the availability of supporting data for this study should be directed to the corresponding author.
Abbreviations
ANCA: antineutrophil cytoplasmic antibody; BVAS: Birmingham Vasculitis Activity Score; CT: computed tomography; EGPA: eosinophilic granulomatosis with polyangiitis; MPO: myeloperoxidase; PR3: proteinase 3
| References | ▴Top |
- Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187-192.
doi pubmed - Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11.
doi pubmed - Sada KE, Kojo Y, Fairburn-Beech J, Sato K, Akiyama S, Van Dyke MK, Mukai I. The prevalence, burden of disease, and healthcare utilization of patients with eosinophilic granulomatosis with polyangiitis in Japan: a retrospective, descriptive cohort claims database study. Mod Rheumatol. 2022;32(2):380-386.
doi pubmed - Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, Khalid S, et al. 2022 American College of Rheumatology/European Alliance of Associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81(3):309-314.
doi pubmed - Koike H, Nishi R, Furukawa S, Mouri N, Fukami Y, Iijima M, Katsuno M. In vivo visualization of eosinophil secretion in eosinophilic granulomatosis with polyangiitis: An ultrastructural study. Allergol Int. 2022;71(3):373-382.
doi pubmed - Koike H, Furukawa S, Mouri N, Fukami Y, Iijima M, Katsuno M. Early ultrastructural lesions of anti-neutrophil cytoplasmic antibody- versus complement-associated vasculitis. Neuropathology. 2022;42(5):420-429.
doi pubmed - Holding S, Fisher VJ, Abuzakouk M. Incidence of PR3- and MPO-ANCA autoantibody specificity changes in ANCA-associated vasculitis. Ann Clin Biochem. 2015;52(Pt 2):297-301.
doi pubmed - De'Oliviera J, Gaskin G, Dash A, Rees AJ, Pusey CD. Relationship between disease activity and anti-neutrophil cytoplasmic antibody concentration in long-term management of systemic vasculitis. Am J Kidney Dis. 1995;25(3):380-389.
doi pubmed - Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623-625.
doi pubmed pmc - Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, Kullman J, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6(1):71.
doi pubmed - Chen M, Kallenberg CG. ANCA-associated vasculitides—advances in pathogenesis and treatment. Nat Rev Rheumatol. 2010;6(11):653-664.
doi pubmed - Jones BE, Yang J, Muthigi A, Hogan SL, Hu Y, Starmer J, Henderson CD, et al. Gene-specific DNA methylation changes predict remission in patients with ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28(4):1175-1187.
doi pubmed pmc - Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68(3):310-317.
doi pubmed - Lardinois OM, Deterding LJ, Hess JJ, Poulton CJ, Henderson CD, Jennette JC, Nachman PH, et al. Immunoglobulins G from patients with ANCA-associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity. PLoS One. 2019;14(2):e0213215.
doi pubmed pmc - Dammacco F, Battaglia S, Gesualdo L, Racanelli V. Goodpasture's disease: a report of ten cases and a review of the literature. Autoimmun Rev. 2013;12(11):1101-1108.
doi pubmed - Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Toumelin PL, French Vasculitis Study G. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore). 2011;90(1):19-27.
doi pubmed - Samson M, Puechal X, Devilliers H, Ribi C, Cohen P, Stern M, Pagnoux C, et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) enrolled in two prospective trials. J Autoimmun. 2013;43:60-69.
doi pubmed - Sinico RA, Bottero P. Churg-Strauss angiitis. Best Pract Res Clin Rheumatol. 2009;23(3):355-366.
doi pubmed - Caminati M, Maule M, Bello F, Emmi G. Biologics for eosinophilic granulomatosis with polyangiitis. Curr Opin Allergy Clin Immunol. 2023;23(1):36-43.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


