| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 5, May 2023, pages 174-178
Native Mitral Valve Endocarditis Caused by a Non-HACEK Gram-Negative Pathogen in a Hemodialysis Patient
Ilire Imeria, Edouard Cubiliera, Maxime Taghavia, Saleh Kaysia, Joelle Nortiera, Maria do Carmo Filomena Mesquitaa, b
aNephrology and Dialysis Department, Brugmann University Hospital, Universite Libre de Bruxelles (ULB), Brussels, Belgium
bCorresponding Author: Maria do Carmo Filomena Mesquita, Nephrology and Dialysis Department, Brugmann University Hospital, Universite Libre de Bruxelles (ULB), B-1020 Brussels, Belgium
Manuscript submitted April 4, 2023, accepted May 18, 2023, published online May 31, 2023
Short title: Non-HACEK Gram-Negative IE in HD
doi: https://doi.org/10.14740/jmc4089
| Abstract | ▴Top |
Infective endocarditis (IE) due to non-HACEK (species other than Hemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella) bacteremia accounts for less than 2% of all IE cases but is proven to be associated with higher mortality, even more so in hemodialysis (HD) patients. Few data are available in the literature concerning non-HACEK Gram-negative (GN) IE in this immunocompromised population with multiple comorbidities. We report the atypical clinical presentation of an elderly HD patient diagnosed with a non-HACEK GN IE, namely E. coli, successfully treated with intravenous (IV) antibiotics. The objective of this case study and related literature was to highlight the limited applicability of the modified Duke criteria in the HD population, as well as the frailty of HD patients that increases their susceptibility to IE due to unexpected microorganisms that could have fatal consequences. The need for a multidisciplinary approach of an IE in HD patients is therefore imperative.
Keywords: Non-HACEK Gram-negative infective endocarditis; E. coli; Modified Duke’s criteria for infective endocarditis; Hemodialysis
| Introduction | ▴Top |
Infections are the second leading cause of mortality in end-stage kidney patients treated with chronic hemodialysis (HD), with an incidence of mortality varying between 12% and 22% [1]. Infective endocarditis (IE) is reported in 2-6% of the HD population, with an incidence ranging from 50 to 60 times higher compared to the general population [2]. The susceptibility of this patient population to IE is due to a multitude of factors such as the presence of a permanent vascular access, a deficient immune system due to uremia, biochemical abnormalities, malnutrition, advanced age, and underlying comorbidities such as type 2 diabetes mellitus (T2DM) [3]. In HD patients, the heart valves are more often calcified due to a disturbed phosphorus-calcium metabolism and premature degeneration of the valves. In approximately 40% of these patients, the mitral valve is affected [4]. In this respect, a highly suspected clinical presentation should prompt the clinician to investigate the possibility of IE diagnosis. We report a case of a successful management of IE in an elderly HD patient with multiple comorbidities.
| Case Report | ▴Top |
Investigations
An 84-year-old male patient presented spontaneously to the emergency room with complaints of respiratory distress at effort and edema of the lower limbs.
This patient’s medical history included Parkinson’s disease, arterial hypertension (AHT), T2DM, moderate aortic valve stenosis (without arguments for a past episode of rheumatic fever), and chronic kidney disease (CKD) stage G4A2 according to the Kidney Disease: Improving Global Outcomes (KDIGO) classification and he had undergone COVID-19 a year earlier. Furthermore, he was previously treated for hepatitis B infection, his human immunodeficiency virus (HIV) status was negative and a Mantoux test excluded tuberculosis (TB). The etiology of CKD was multifactorial (AHT, T2DM) and partly attributed to the complications following intestinal ischemia which was in relation with three episodes of volvulus for which the patient underwent a sigmoidectomy and had a permanent colostomy.
Drug medications included amlodipine 10 mg q.d., cholecalciferol 25,000 IU/week, sodium bicarbonate 1 g b.i.d., pegylated erythropoietin (Mircera©) 50 µg/month, sitagliptin 25 mg q.d., rasagiline 1 mg q.d., pantoprazole 40 mg q.d. and tamsulosin 0.4 mg q.d.
On admission, blood biological parameters showed acutization of pre-existing CKD (AKIN stage 1) (serum creatinine of 5.44 mg/dL with baseline at around 3.80 mg/dL), no significant elevation of the C-reactive protein (CRP) and elevated N-terminal pro b-type natriuretic peptide (NT-proBNP) (4,638 ng/L) (Fig. 1), suggesting the diagnosis of heart failure (HF) secondary to volume overload. A chest computed tomography (CT) showed bilateral pleural effusion (Fig. 2a). Diuresis was preserved, and a transient bladder catheterization was initiated to monitor the response to diuretic therapy. The patient was admitted into the nephrology ward for management of a suspected cardio-renal syndrome.
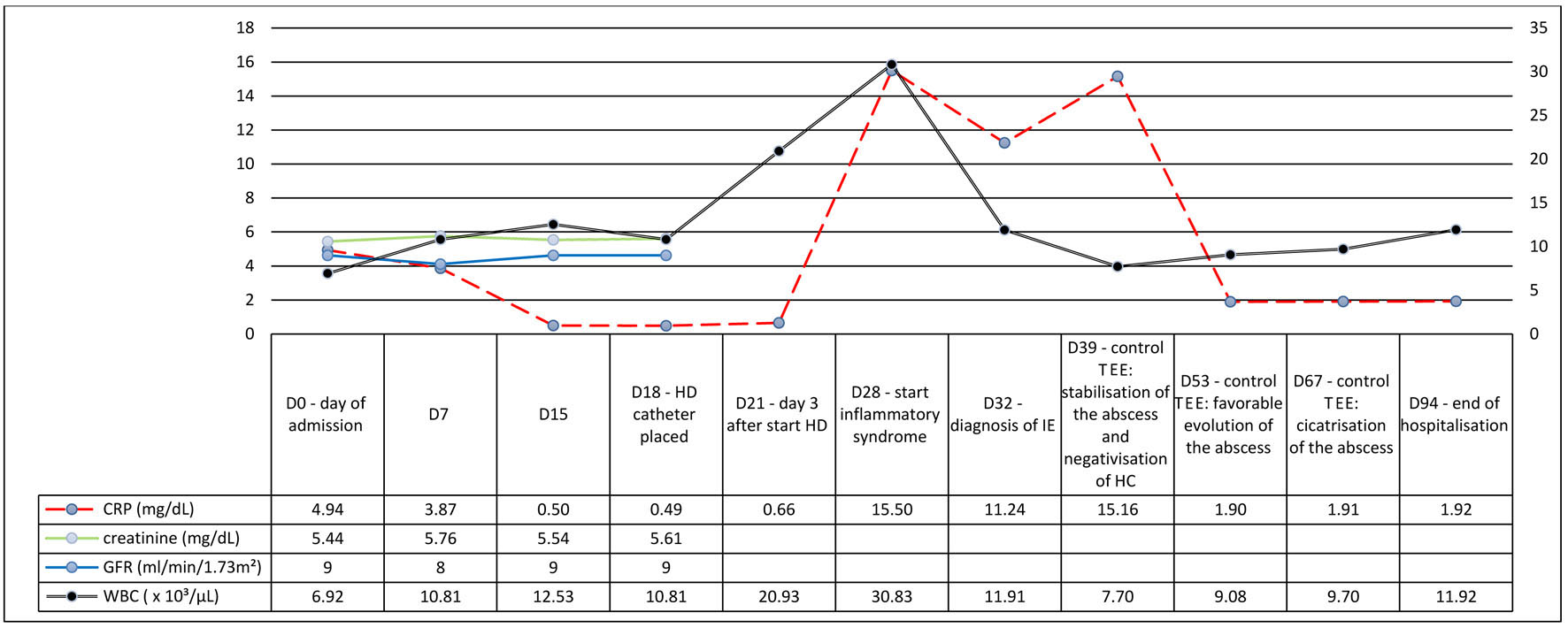 Click for large image | Figure 1. Evolution of the blood biological parameters. |
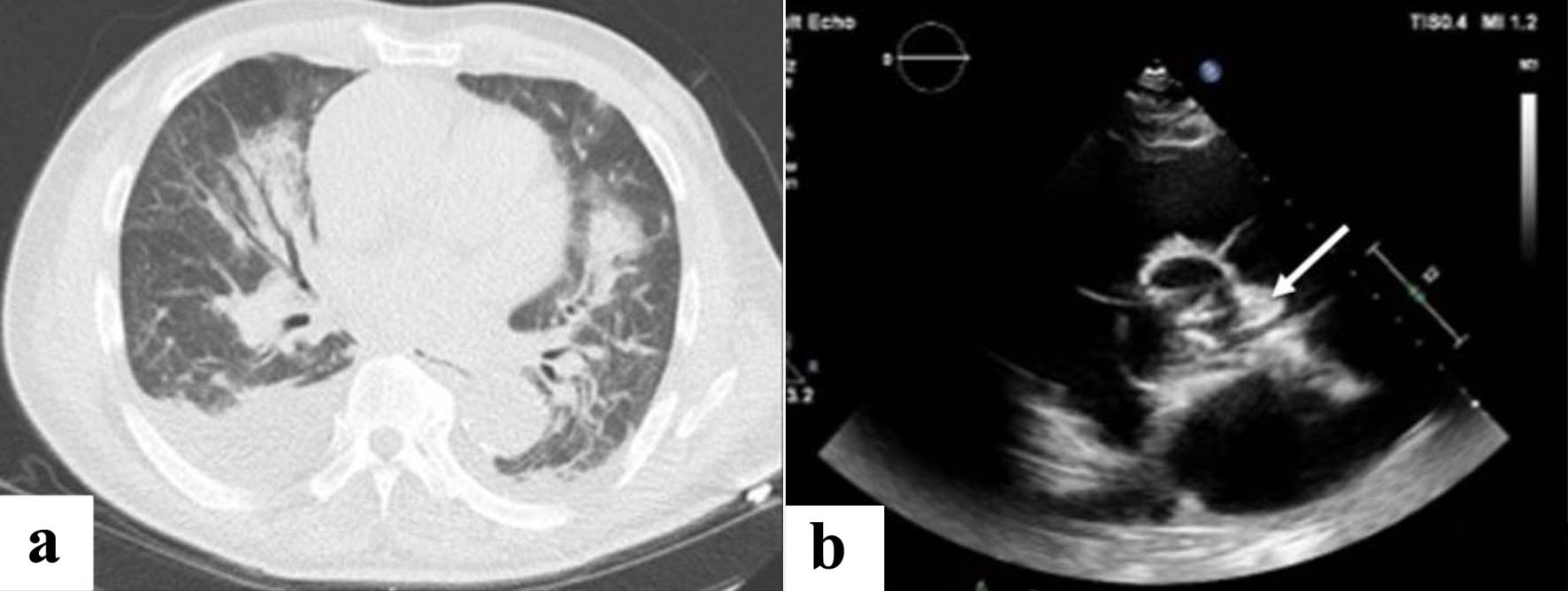 Click for large image | Figure 2. (a) Chest CT scan upon admission (D0). (b) TEE at day 32 confirming an infective endocarditis. Arrow shows a vegetation (oscillating intracardiac mass on the mitral, described by the cardiologist as an abscess). CT: computed tomography; TEE: transesophageal echocardiography. |
Diagnosis
The patient’s clinical course was marked by an initial improvement of the cardiorespiratory status due to titration intravenous (IV) furosemide. However, the renal function continued to deteriorate, leading to the placement of a tunnelled hemodialysis catheter (Hickmann©) at day (D) 18 with subsequent initiation of HD.
At D28, 3 days after catheter placement, a rise in the inflammatory markers was seen (white blood cells at 30.83 × 103/µL and CRP increased to 155 mg/L) without any clinical sign of infection (i.e., no tachycardia, no tachypnoea, no fever). Both urine analysis and two pairs of peripheral blood cultures, collected daily from D28 until D36, were positive for E. coli with production of extended spectrum beta-lactamase (ESBL), leading to prompt initiation of temocillin 2 g q.d. IV on D28. Blood samples through the HD catheter remained negative, thus excluding this catheter as source of bacteremia. Four days later, a transthoracic echocardiography (TTE) showed a vegetation of the anterior mitral valve leaflet along with an abscess of the mitral-aortic junction of approximately 9 mm in diameter (Fig. 2b). Third-degree atrioventricular (AV) block was also detected during the examination, resulting in transfer to the cardiology ward for monitoring by a temporary transcutaneous pacing. A transesophageal echocardiography (TEE) confirmed the presence of the vegetation as an oscillating intracardiac mass on the mitral valve, considered by the cardiologist as an abscess and therefore a case of IE.
Treatment, follow-up and outcomes
Regular TTE and TEE were performed in order to follow the size of the vegetations and the abscess under antibiotic therapy. Antibiotics were continued for a total of 3 months from D28. Restoration of sinus rhythm was also observed during the antibiotic therapy, thus avoiding the need of a pacemaker. The patient was discharged from the hospital at D94 and transferred to a rehabilitation unit. Currently, he is dialyzed through the same HD catheter and shows no further complications. An overview of the evolution of the blood biological parameters is shown in Figure 1.
| Discussion | ▴Top |
Diagnosing IE in HD patients can be a clinical dilemma. As demonstrated by the present case, using the modified Duke criteria (Table 1) [5] complicates the diagnosis of IE in HD. According to these criteria, our patient’s case has to be categorized as possible IE because he had one major criterion, namely, the presence of a vegetation and abscess, well visualized in a patient despite having calcified mitral and aortic valves. Moreover, he had only two minor criteria, i.e., a predisposing heart condition (calcified cardiac valves) and several positive blood cultures for microorganisms that did not meet major criteria (E. coli is not considered to be a typical microorganism). Nevertheless, because of the total disappearance of lesions on control echocardiography, the improvement of the patient’s clinical and biological parameters after antibiotic therapy, one could categorize our patient as having IE.
 Click to view | Table 1. Modified Duke Criteria for the Diagnosis of IE [5] |
As reviewed by Nucifora et al, the main causative organism in up to 80% of cases of IE in the HD population is found to be S. aureus, followed by Streptococcus spp., Enterococcus spp. and bacteria of the HACEK group [3]. The high nasal carriage of methicillin-sensitive S. aureus and the recurrent and longer stays of HD patients in health institutions explain the high frequency of Gram-positive bacteria in this population. In contrast, IE due to non-HACEK bacteria and especially by E. coli like in our patient, is rare as shown by the study of Morpeth et al, where they saw E. coli to be the culprit bacteria in 0.5% of the 2,761 studied patients [6]. The problem of IE related to Gram-negative (GN) non-HACEK bacilli in HD patients is even rarer, hence the decision to report about our patient’s case.
It is well known that the vascular access, whether it is a hemocatheter or an arterio-venous fistula, remains the main entry point for bacteremia, but this is a fact on which we should not blindly focus on. Other sources of infection should also be considered, such as urinary tract infections, dental problems, diverticulosis, cardiac devices, etc. [7]. Our patient had a bladder catheter which was placed upon admission (D0) for the follow-up of diuresis, resulting in a bacteriuria and bacteremia from urinary origin. Urinary catheters, placed for whatever reason, are not innocuous.
Historically, non-HACEK IE was associated with IV drug users. Morpeth and Falcone identified the main risk factors for an IE due to a GN bacteremia: the presence of implanted endovascular devices (IEDs), state of immunosuppression of the patient, T2DM and genitourinary tract infection. In-hospital mortality and long-term survival were then rather determined by the multi-drug resistance of the organism [6, 8].
CKD and HD patients are to be considered as immunocompromised [1, 9]. Due to the presence of uremia, there is an increased state of inflammation, which enhances susceptibility to IE [3, 6, 8, 10]. Our patient was an elderly patient known with longstanding CKD and suffering from multiple comorbidities who was carrying a recently placed indwelling hemocatheter. He was therefore at high risk to develop IE. In HD patients, the typical signs of inflammation and infection may be obscured. Pyrexia may be absent in 45-70% of cases; however, as a minor Duke criterion, it has an important negative predictive value [3]. It is not clear if the modified Duke’s criteria are validated for the HD population. Possible suggestion to increase its applicability would be the immediate heart imaging in the presence of bacteremia, even in case of, as stated by the modified Duke’s criteria, an atypical organism as in the case of our patient.
Furthermore, due to a disturbed phosphorus-calcium metabolism, they are also prone to premature degeneration of the valves (up to 10 years earlier compared to the normal population) [4]. This could make the visualization of vegetation or abscess on echocardiography less evident for the cardiologist. This means that the observed vegetation in our patient could have been there before the clinical and biochemical manifestations of IE.
IE due to E. coli can also possibly be explained by certain strains of E. coli possessing the right virulence factors. Russo et al coined the term ExPEC (extra-intestinal pathogenic E. coli), belonging to E. coli phylogenetic group B2, to indicate those strains causing extra-intestinal infections. They acquired the capacity to adhere and invade tissues as well as to escape the host’s immunity [11]. In our patient however, it was not known if the cultured E. coli was from this group, neither the source of E. coli. Although both urine samples and blood samples were positive for the same pathogen, a bacterial translocation of the digestive tract cannot be excluded in our patient. Risk factors associated with this strain are increased health care contact as well as the aforementioned risk factors. IE should thereby be a possible differential diagnosis in an HD patient with E. coli bacteremia.
Following a consensus meeting with nephrologists, infectious disease specialists and surgeons, it was decided not to perform surgery in our patient because of the high risk for cardiac intervention, to opt for a conservative treatment and to continue antibiotic therapy according to the European Society of Cardiology (ESC) guidelines [12]. Actually, after nearly 1 year, our patient is doing well on HD. Thereby, we wish to emphasize the importance of multidisciplinary discussion in all cases of IE.
Conclusions
IE has a high mortality rate in HD patients and its clinical presentation can be insidious. In every HD patient presenting with bacteremia, the possibility of IE should be raised. TTE, and if not sufficient, TEE should then be performed as soon as possible. The outmost precision is necessary, as heavily calcified heart valves make visualization of the vegetations difficult. The modified Duke criteria for diagnosis of IE may not always be applicable in HD population. Clinicians caring for HD patients should be alert to unusual bacterial causes of IE such as E. coli and atypical clinical presentation. Although vascular access is the most important source of infection in HD, clinicians should be aware of other sources of infections, such as the genito-urinary or digestive tract, from where rare microbes like E. coli (non-HACEK GN group) can adhere to the heart valves causing sometimes fatal IE. The management of HD patients whose multiple comorbidities have rendered them fragile is ultimately a multidisciplinary undertaking. The available literature concerning non-HACEK IE, especially caused by a GN species, is sparse in HD population. Hence, studies dealing only with this population could be difficult if we do not have an adequate sample size. Clinicians should be encouraged to publish their data, even though they originate from case reports or case series.
Learning points
Every case of bacteremia in HD patient should evoke the possibility of IE with the prompt investigation by TTE and, if necessary, by TEE. Other sources of infection beside the vascular access in HD patients should be considered such as the genito-urinary and digestive tract. IE management requires a multidisciplinary approach as the number of comorbidities in HD patients is high.
Acknowledgments
None to declare.
Financial Disclosure
No funds were obtained for this work.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Written informed consent was obtained from the patient by the authors.
Author Contributions
All authors contributed in collecting the data and writing the manuscript. MM and JN revised the manuscript. All authors were involved in the preparation of this work.
Data Availability
Data generated during the current case are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Powe NR, Jaar B, Furth SL, Hermann J, Briggs W. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int. 1999;55(3):1081-1090.
doi pubmed - Bentata Y. Physiopathological approach to infective endocarditis in chronic hemodialysis patients: left heart versus right heart involvement. Ren Fail. 2017;39(1):432-439.
doi pubmed pmc - Nucifora G, Badano LP, Viale P, Gianfagna P, Allocca G, Montanaro D, Livi U, et al. Infective endocarditis in chronic haemodialysis patients: an increasing clinical challenge. Eur Heart J. 2007;28(19):2307-2312.
doi pubmed - Hoevelmann J, Mahfoud F, Lauder L, Scheller B, Bohm M, Ewen S. Valvular heart disease in patients with chronic kidney disease. Herz. 2021;46(3):228-233.
doi pubmed - Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr., Ryan T, Bashore T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633-638.
doi pubmed - Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, Nacinovich F, et al. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147(12):829-835.
doi pubmed - Rafael DGH, Anabel C la TN, Alfonso AV, Jesus CGM de, Berenice GJO, Edgar EB, Eloisa RRG, et al. Infective endocarditis in end-stage renal disease patients in developing countries: what is the real problem? In: Firstenberg MS (Ed), contemporary challenges in endocarditis. IntechOpen, Rijeka. 2016.
doi - Falcone M, Tiseo G, Durante-Mangoni E, Ravasio V, Barbaro F, Ursi MP, Pasticci MB, et al. Risk factors and outcomes of endocarditis due to non-HACEK Gram-Negative Bacilli: data from the prospective multicenter Italian endocarditis study cohort. Antimicrob Agents Chemother. 2018;62(4):e02208-e02217.
doi pubmed pmc - Eleftheriadis T, Liakopoulos V, Leivaditis K, Antoniadi G, Stefanidis I. Infections in hemodialysis: a concise review - Part 1: bacteremia and respiratory infections. Hippokratia. 2011;15(1):12-17.
pubmed pmc - Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893.
doi pubmed - Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181(5):1753-1754.
doi pubmed - Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


